Ethyleneimine
- CAS NO.:151-56-4
- Empirical Formula: C2H5N
- Molecular Weight: 43.07
- MDL number: MFCD00039669
- EINECS: 205-793-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
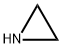
What is Ethyleneimine?
Description
Ethyleneimine is a colourless liquid with an ammonia-like smell or pungent odour. It is highly flammable and reacts with a wide variety of materials. Ethyleneimine is used in polymerisation products, as a monomer for polyethyleneimine and as a comonomer for polymers, for example, with ethylenediamine. Polymerised ethyleneimine is used in paper, textile chemicals, adhesive binders, petroleum, refining chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid flocculants, and surfactants. Ethyleneimine readily polymerises, and it behaves like a secondary amine. Ethyleneimine is highly caustic, attacking materials such as cork, rubber, many plastics, metals, and glass except those without carbonate or borax. It polymerises explosively on contact with silver, aluminium, or acid. The activity of ethyleneimine is similar to that of nitrogen and sulphur mustards. Ethyleneimine is used as an intermediate in the production of triethylenemelamine.
Chemical properties
colourless liquid with an ammonia-like smell
Chemical properties
Ethyleneimine is a colorless liquid with an ammonia-like smell or pungent odor. It is highly flammable and reacts with a wide variety of materials. Ethyleneimine is used in polymerization products, as a monomer for polyethyleneimin, and as a comonomer for polymers, e.g., with ethylenediamine. Polymerized ethylenimine is used in paper, textile chemicals, adhesive binders, petroleum, refi ning chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid fl occulants, and surfactants. Ethyleneimine readily polymerizes, and it behaves like a secondary amine. Ethyleneimine is highly caustic, attacking materials such as cork, rubber, many plastics, metals, and glas except those without carbonate or borax. It polymerizes explosively on contact with silver, aluminum, or acid. The activity of ethyleneimine is similar to that of nitrogen and sulfur mustards. Ethyleneimine is used as an intermediate in the production of triethylenemelamine. Polymerized ethyleneimine is used in paper, textile chemicals, adhesive binders, petroleum, refi ning chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid fl occulants, and surfactants
Chemical properties
Ethyleneimine is a colorless volatile liquid with an ammoniacal odor.
Chemical properties
The strained three-membered ring structure of ethyleneimine readily undergoes ring-opening reactions, which are catalyzed by acids and occur at moderate temperatures. Ethyleneimine is easily polymerized at elevated temperatures in the presence of catalytic amounts of acid. Reactions with ammonia and primary orsecondary amines in the presence of catalytic amounts of acids yield ethylenediamines; reactions with carboxylic acids yield 2-aminoethyl esters.
Ethyleneimine is also a highly reactive secondary amine, and undergoes a large number of reactions under neutral or basic conditions which yield products with the three-membered ring intact. Addition reactions occur with acyl halides, alkyl and substituted alkyl halides, aryl halides, and other halogen-containing compounds. Ethyleneimine forms adducts with aldehydes, ketones, and olefinic compounds (Ham 1978).
Physical properties
Clear, colorless, very flammable liquid with a very strong ammonia odor. Odor threshold concentration is 1.5 ppm (quoted, Amoore and Hautala, 1983).
The Uses of Ethyleneimine
Ethyleneimine is used to manufacture triethylenemelamine and is used in its polymeric form in paper and textile chemicals, adhesive binders, petroleum-refining chemicals, fuels and lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid flocculants, and surfactants.
The Uses of Ethyleneimine
In the manufacture of triethylenemelamine.
The Uses of Ethyleneimine
Ethylenimine is used in the manufacture oftriethylenemelamine and other amines.
Production Methods
Industrial quantities are made with monoethanolamine via a two-step chemical dehydration process using sulphuric acid and sodium hydroxide, or by reacting 1,2-dichloroethane with ammonia. The U.S. production in 1978 was over 1500 metric tons (Ham 1978).
Definition
ChEBI: Aziridine is a saturated organic heteromonocyclic parent, a member of aziridines and an azacycloalkane. It has a role as an alkylating agent. It is a conjugate base of an aziridinium.
Origin
Ethylenimine was first prepared in 1888 by GABRIEL, who mistakenly called it vinylamine. He prepared the ethylenimine by reacting 2-bromoethylamine hydrobromide with silver oxide or potassium hydroxide.
General Description
A clear colorless liquid with an ammonia-like odor. Flash point 12°F. Less dense than water. Flammable over a wide range of vapor-air concentrations. Vapors irritate the skin, eyes, nose, and throat. May be toxic by prolonged inhalation, skin absorption, or ingestion. Carcinogenic. Vapors heavier than air. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
ETHYLENEIMINE vapors are not inhibited and may form polymers in vents or flame arresters, resulting in stopping of the vents. Produces toxic oxides of nitrogen during combustion. Reacts with sodium hypochlorite and other chlorinating agents to give the explosive compound 1-chloroazidine. Decomposes if heated under pressure. or else hazardous polymerization may occur. Incompatible with silver or aluminum, which induce polymerization May polymerize explosively upon contact with acids. Polymerization is catalyzed by carbon dioxide [EPA, 1998].
Hazard
Corrosive, absorbed by skin, causes tumors; exposure should be minimized; a carcinogen. Dangerous fire and explosion hazard, flammable limits in air 3.6–46%. Toxic by skin absorption; possible carcinogen.
Health Hazard
Ethyleneimine is classified as extremely toxic with a probable oral lethal dose of 5-50 mg/kg which is approximately 7 drops to 1 teaspoonful for a 70 kg (150 lb.) person. Ethyleneimine gives inadequate warning when over-exposure is by inhalation or skin absorption. It is a severe blistering agent, causing third degree chemical burns of the skin. Also, it has a corrosive effect on mucous membranes and may cause scarring of the esophagus. It is corrosive to eye tissue and may cause permanent corneal opacity and conjunctival scarring. Severe exposure may result in overwhelming pulmonary edema. Renal damage has been described. Hemorrhagic congestion of all internal organs has been observed.
Health Hazard
Exposures to ethyleneimine cause adverse health effects and poisoning. Ingestion/swallowing, inhalation, or absorption through exposures to skin cause severe irritation, blisters, severe deep burns, and effects of sensitization. Ethyleninime is corrosive to the eye tissue and may cause permanent corneal opacity and conjunctival scarring, severe respiratory tract irritation, and effects of infl ammation in workers. Ethyleneimine is a severe blistering agent, causing third degree chemical burns of the skin. The symptoms of toxicity include, but are not limited to, cough, dizziness, headache, labored breathing, nausea, vomiting, tearing and burning of the eyes, sore throat, nasal secretion, bronchitis, shortness of breath, laryngeal edema, pronounced changes of the trachea and bronchi of lungs. Ethyleneimine with its corrosive effects cause injury to the mucous membranes and acute oral exposure may cause scarring of the esophagus in humans. The onset of symptoms and health effects caused by ethyleneimine depends on exposure concentration.
Health Hazard
Ethylenimine is highly toxic by all exposure routes. Airborne exposure causes conjunctivitis, respiratory tract irritation, edema, and albuminuria (Weightman and Hoyle 1964), with possible damage to liver and kidneys; vomiting and other CNS effects may occur at high exposures. Dermal contact to ethylenimineproduces severe irritation, with lesions which are slow to heal. Ethylenimines are also skin sensitizers (Garabrant 1985; Cofield et al 1985). There are no reports which indicate potential reproductive effects or increased risk for cancer in humans exposed to ethylenimine.
Health Hazard
Ethylenimine is a highly poisonous com pound and a severe irritant to the skin, eyes,and mucous membranes. A 20–25% aqueoussolution on contact with the eyes can causecorneal opacity and loss of vision. Skin con tact with the pure liquid or its concentratedsolution can produce severe burns and skinsensitization.
Ethylenimine is highly toxic by all routesof exposure. Inhalation of its vapors cancause eye, nose, and throat irritations anddifficulty in breathing. The toxic symptomsnoted from repeated exposures include chestcongestion, delayed lung injury, vomiting,hemorrhage, and kidney damage. An 8-hourexposure to 100 ppm in air proved fatal torabbits. The symptoms from acute oral toxic ity in humans may include nausea, vomiting,dizziness, headache, and pulmonary edema.Chronic toxic effects may result in kidneyand liver damage. The acute oral LD50 valuein rats was 15 mg/kg. Ethylenimine is alsoabsorbed through the skin, exhibiting poi soning effects similar to those of acute oraltoxicity.
It exhibited reproductive toxicity in ani mals, indicating paternal effects and specificdevelopmental abnormalities in the centralnervous system, eyes, and ears. Ethylenimineis a mutagen, testing positive in the histi dine reversion–Ames test as well as in thein vitro cytogenetics–human lymphocyte,Drosophila melanogaster–reciprocal translo cation, Saccharomyces cerevisiae gene con version, and other mutagenic tests. Animalstudies show sufficient evidence of carcino genicity. It may cause cancers in the lungsand liver.
Fire Hazard
Irritating vapors are generated when heated. Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. May polymerize in fires with evolution of heat and container rupture. Runoff to sewer may create fire or explosion hazard. Ethyleneimine vapors are not inhibited and may form polymers in vents or flame arresters, resulting in stopping of the vents. Toxic oxides of nitrogen are produced during combustion. Upon treatment with sodium hypochlorite, Ethyleneimine gives off the explosive compound 1-chloroazidine. Avoid acids, sodium hypochlorite. If heated under pressure, instability may result. Hazardous polymerization may occur. Avoid contact with silver or aluminum. Explosive polymerization may occur upon contact with acids. Polymerization is catalyzed by carbon dioxide.
Flammability and Explosibility
Highly flammable
Chemical Reactivity
Reactivity with Water: Mild reaction, non-hazardous; Reactivity with Common Materials: Contact with silver or aluminum may cause polymerization; Stability During Transport: Stable unless heated under pressure; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Explosive polymerization can occur when in contact with acids; Inhibitor of Polymerization: None used.
Industrial uses
Approximately 50% of ethylenimine produced in the U.S. is polymerized to polyethyleneimine, used as a flocculant in water treatment, and as a wet-strength additive in the textile and paper industries. Polyethylenimine is also used in various adhesives and coatings and to laminate plastic films to paper, other cellulose materials, and metal foils for making cartons in the food industry. The adhesion properties of acrylic latex paints are improved by reaction of acid groups with ethylenimine. Ethylenimines are utilized in the textile industry to improve durability, crease resistance, flame resistance, and dyeing properties. Other uses are found in ion-exchange resin synthesis, in electroplating, as a rocket propellant binder, as a lubricating oil dispersant, and as a hardening agent in the preparation of photographic films. Ethylenimine is used in the manufacture of triethylene melamine, a cancer chemotherapy drug; various ethylenimines are used as insect chemosterilant agents for pest control (Ham 1978).
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Other experimental reproductive effects. Poison by ingestion, skin contact, inhalation, and intraperitoneal routes. Human mutation data reported. A skin, mucous membrane, and severe eye irritant. An allergc sensitizer of skin. Causes opaque cornea, keratoconus, and necrosis of cornea (experimentally). Has been known to cause severe human eye injury. Drinking of carbonated beverages is recommended as an antidote to ths material in stomach. A very dangerous fire and explosion hazard when exposed to heat, flame, or oxidzers. Reacts violently with acids, aluminum chloride + substituted anilines, acetic acid, acetic anhydride, acrolein, acrylic acid, allyl chloride, CS2, Cl2, chlorosulfonic acid, epichlorohydrin, glyoxal, HCl, HF, HNO3, oleum, P-propiolactone, Ag, NaOCl, H2SO4, vinyl acetate. Reacts with chlorinating agents (e.g., sodum hypochlorite solution) to form the explosive 1 chloroaziridine. Reacts with silver or its alloys to form explosive silver derivatives. Dangerous; heat and/or the presence of catalytically active metals or chloride ions can cause a violent exothermic reaction. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Ethyleneimine is used in production of binding agents; formation of plastics; and improving paper strength; in many organic syntheses; as an intermediate and monomer for fuel oil and lubricating refining. The polymerization products, polyethyleneimines, are used as auxiliaries in the paper industry and as flocculation aids in the clarification of effluents. It is also used in the textile industry for increasing wet strength, flame-, water-, shrinkproofing, and stiffening
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least45 min, occasionally lifting upper and lower lids. Seek medical attention immediately since eye splashes can causesevere illness or death. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray
Carcinogenicity
The carcinogenicity of ethyleneimine was evaluated in two strains of mice, and both gave positive results. Groups of 18 male and 18 female mice of B6C3F1 or B6AKR strains were treated orally (initially by gavage, then in the diet) from age 7 days through 77–78 weeks. The time-weighted average (TWA) dose was about 1.8 mg/kg/day. The incidence of hepatomas and lung adenomas was significantly elevated in both strains and sexes. In B6C3F1 mice, the incidence of hepatomas and pulmonary adenomas was 15/17 and 15/17 in males and 11/15 and 15/15 females, respectively. In the B6AKR strain, hepatomas and adenomas occurred in 9/16 and 12/16 males and in 2/11 and 10/11 females, respectively. In the control groups, hepatomas were 8/79 and 0/87 in male and female B6C3F1 mice and 5/90 and 1/82 in male and female B6AKR mice. The respective incidence of pulmonary adenomas was 5/79, 3/87, 10/90, and 3/82. The incidence of hepatomas and pulmonary adenomas (reported as combined tumors) was significantly (p<0.01) elevated.
Environmental Fate
Photolytic. The vacuum UV photolysis (λ = 147 nm) and γ radiolysis of ethylenimine resulted
in the formation of acetylene, methane, ethane, ethylene, hydrogen cyanide, methyl radicals, and
hydrogen (Scala and Salomon, 1976). Photolysis of ethylenimine vapor at krypton and xenon lines
yielded ethylene, ethane, methane, acetylene, propane, butane, hydrogen, ammonia, and ethyleneimino
radicals (Iwasaki et al., 1973).
Chemical/Physical. Polymerizes easily (Windholz et al., 1983). Hydrolyzes in water forming
ethanolamine (HSDB, 1989). The estimated hydrolysis half-life in water at 25 °C and pH 7 is 154
d (Mabey and Mill, 1978).
Metabolism
When male Dow-Wistar rats were injected intraperitoneally with [14C]-ethylenimine (80mug), approximately half of the dose was excreted in the urine (Wright and Rowe 1967). The major portion of the radioactivity in the urine consisted of unidentified products, although a small amount was excreted unchanged. A small portion, 3-5%, was expired as 14C02, and 1-3% was expired as a volatile, basic material, probably ethylenimine, during 24 h. Significant amounts of radioactivity were accumulated in liver, intestines, cecum, spleen, and kidneys. After 24 h, tissue radioactivity became constant and essentially unavailable for further metabolism. The aziridine ring of drugs is readily cleaved by microsomal enzymes, possibly with intermediate formation of an N-oxide (Oelschlager and Al Shaik 1985).
storage
(1) Color Code—Red: Flammability Hazard: Storein a flammable liquid storage area or approved cabinetaway from ignition sources and corrosive and reactivematerials. (2) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Ethyleneimine must be inhibitedand stored to avoid contact with strong acids (such ashydrochloric, sulfuric, and nitric) and oxidizers (such asperchlorates, peroxides, permanganates, chlorates, andnitrates) since violent reactions occur. Store in tightlyclosed containers in a cool, well-ventilated area. Sources ofignition, such as smoking and open flames, are prohibitedwhere ethyleneimine is handled, used, or stored. Metal containers involving the transfer of 5 gallons or more of ethyleneimine should be grounded and bonded. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof ethyleneimine. Wherever ethyleneimine is used, handled,manufactured, or stored, use explosion-proof electricalequipment and fittings. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045
Shipping
UN1185 Ethyleneimine, stabilized, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Inhalation Hazard Zone A. PGI
Purification Methods
Redistil it in an Ar or N2 atmosphere in a fume hood, and store it over KOH in sealed bottles in a refrigerator. Commercial aziridine has been dried over sodium and distilled from the metal through an efficient column before use [Jackson & Edwards J Am Chem Soc 83 355 1961, Wenker J Am Chem Soc 57 2328 1935]. It is a weaker base than Me2NH (pK2 5 10.87) but is caustic to the skin. It should not be inhaled, causes inflammation of the eyes, nose and throat, and one may become sensitized to it. It is soluble in H2O, has an ammoniacal smell and reacts with CO2. Pure aziridine is comparatively stable but polymerises in the presence of traces of H2O and is occasionally explosive in the presence of acids. CO2 is sufficiently acidic to cause polymerisation (forms linear polymers) which is not free radical promoted. It is stable in the presence of bases. The violet 2:1 Cu complex crystallises from EtOH containing a few drops of aziridine and adding Et2O, and has m 142o(dec). The picrate has m 142o. [O'Rourke et al. J Am Chem Soc 78 2159 1956.] It has also been dried over BaO and has been distilled from sodium under nitrogen. [Allen et al. Org Synth Coll Vol IV 433 1963, Beilstein 20 III/IV 1.] TOXIC.
Toxicity evaluation
Ethyleneimine is an extremely reactive alkylating agent that undergoes ring-opening reactions with cellular nucleophiles.
Incompatibilities
May form explosive mixture with air. Ethyleneimine is a medium strong base. Contact with acids, aqueous acid conditions, oxidizers, aluminum, or carbon dioxide may cause explosive polymerization. Explosive silver derivatives may be formed with silver alloys e.g., silver solder). Self-reactive with heat or atmospheric carbon dioxide. May accumulate static electrical charges, and may cause ignition of its vapors. Attacks rubber, coatings, plastics, and chemically active metals. Ethyleneimine vapors are not inhibited and may form polymers in vents or flame arresters, resulting in stopping of the vents.
Waste Disposal
Controlled incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions
Precautions
During use of ethyleninime, students and occupational workers should wear protective equipment, such as gloves, safety glasses, and should have good ventilation. Ethyleninime should be handled as a carcinogen. Ethyleninime vapor/air mixtures are explosive and pose a risk of fi re and explosion on contact with acid(s), oxidants.
Properties of Ethyleneimine
| Melting point: | -78°C |
| Boiling point: | 56°C |
| Density | 0,83 g/cm3 |
| vapor pressure | 160 at 20 °C, 250 at 30 °C (quoted, Verschueren, 1983) |
| refractive index | nD25 1.412 |
| Flash point: | -11°C |
| solubility | miscible with water and virtually all organic solvents |
| form | Colorless liquid |
| pka | 8.01(at 25℃) |
| Odor | Fishy; ammoniacal. |
| Water Solubility | miscible |
| Henry's Law Constant | 1.33(x 10-7 atm?m3/mol) at 25 °C (quoted, Mercer et al., 1990) |
| Exposure limits | TLV-TWA (skin) 0.5 ppm (~1 mg/m3)
(ACGIH, OSHA, and MSHA); Poten tial Human Carcinogen in the workplace
(OSHA), Potential Carcinogen (NIOSH). |
| Dielectric constant | 18.3(25℃) |
| Stability: | Highly flammable. Reacts with a wide variety of materials. |
| CAS DataBase Reference | 151-56-4(CAS DataBase Reference) |
| IARC | 2B (Vol. 9, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Ethylenimine(151-56-4) |
| EPA Substance Registry System | Aziridine (151-56-4) |
Safety information for Ethyleneimine
Computed Descriptors for Ethyleneimine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[(2-Chlorophenyl)-N-(methylimino)methyl]cyclopentanol hydrochloride](https://img.chemicalbook.in/CAS/GIF/90717-16-1.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1