Triethylborane
Synonym(s):Triethylboron
- CAS NO.:97-94-9
- Empirical Formula: C6H15B
- Molecular Weight: 97.99
- MDL number: MFCD00009022
- EINECS: 202-620-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-12 09:25:32
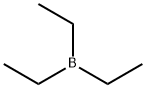
What is Triethylborane?
Chemical properties
Clear colourless to light amber solution. Miscible with most organic solvents; immiscible with water.
The Uses of Triethylborane
Triethylborane reacted with 8-hydroxyquinoline to synthesize three 8--?hydroxyquinolato (q) boron compounds B(C2H5)?2q (1)?, BPh2q (2)?, and B(2-?naph)?2q (3).
The Uses of Triethylborane
Reagent in organic syntheses blocking agent and radical initiator.
The Uses of Triethylborane
It is used in mixtures with triethylaluminumas hypergolic igniters in rocket propulsionsystems.
Synthesis Reference(s)
The Journal of Organic Chemistry, 51, p. 427, 1986 DOI: 10.1021/jo00354a002
Hazard
Flammable, dangerous fire risk, ignites spontaneously in air. Reacts violently with water and oxidizing materials. Toxic by inhalation, strong irritant.
Health Hazard
Triethylboron is a toxic substance. The toxicsymptoms from chronic exposure to thiscompound are not reported. Skin contact canburn tissues.
Fire Hazard
Triethylborane is a pyrophoric substance, igniting spontaneously on exposure to air, chlorine, or bromine. Explosion may result when mixed with oxygen. Contact with ozone, peroxides and other oxidants can cause explosion. Its concentrated solutions can be pyrophoric. Flash point of 1 M solution in hexane is -23°C ( -9°F), while that in tetrahydrofuran is -17°C (1°F) (Aldrich 1996). It decomposes explosively when mixed with water.
Flammability and Explosibility
Pyrophoric
Safety Profile
Poison by ingestion and intraperitoneal routes. Mildly toxic by inhalation. Animal experiments show that the vapor is a poison which causes pulmonary irritation and convulsions. A very dangerous fire hazard by spontaneous chemical reaction with oxichzers. Spontaneously flammable in air. Explodes in oxygen atmospheres. Hypergolic reaction with triethylaluminum. Ignites on contact with chlorine, bromine, or other halogens. Will react with water or steam to produce toxic and flammable vapors. To fight fire, do NOT use halogenated extinguishing agents. When heated to decomposition or upon contact with air it emits toxic acrid smoke and irritating fumes. See also BORANES and BORON COMPOUNDS.
Purification Methods
It distils at 56-57o/220mm. It can also be purified via its ammonia addition complex which is distilled in a high vacuum, decomposed with dry HCl, and the Et3B is distilled out. It is commercially available as a 15% solution in hexane or as 1M solution in hexane. [Brown J Am Chem Soc 67 376 1945, Bamford et al. J Chem Soc 471 1946, Lin et al. J Organomet Chem 312 277 1986, Beilstein 4 III 1957, 4 IV 4359.]
Properties of Triethylborane
| Melting point: | -92°C |
| Boiling point: | 95°C |
| Density | 0.865 g/mL at 25 °C |
| vapor pressure | 67hPa at 25℃ |
| refractive index | n |
| Flash point: | 1 °F |
| storage temp. | 0-6°C |
| form | Liquid |
| color | Colorless to light amber |
| Water Solubility | Decomposes |
| Sensitive | Air Sensitive |
| Merck | 14,9668 |
| BRN | 1731462 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| CAS DataBase Reference | 97-94-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Borane, triethyl-(97-94-9) |
| EPA Substance Registry System | Borane, triethyl- (97-94-9) |
Safety information for Triethylborane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H250:Pyrophoric liquids; Pyrorophoric solids H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Triethylborane
| InChIKey | LALRXNPLTWZJIJ-UHFFFAOYSA-N |
Triethylborane manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 97-94-9 Triethyl Borane (1.0 M in THF)View Details
97-94-9 Triethyl Borane (1.0 M in THF)View Details
97-94-9 -
 97-94-9 Triethylborane solution, 1M in THF 99%View Details
97-94-9 Triethylborane solution, 1M in THF 99%View Details
97-94-9 -
 Triethylborane CAS 97-94-9View Details
Triethylborane CAS 97-94-9View Details
97-94-9 -
 Triethylborane (ca. 11% in Tetrahydrofuran, ca. 1mol/L) CAS 97-94-9View Details
Triethylborane (ca. 11% in Tetrahydrofuran, ca. 1mol/L) CAS 97-94-9View Details
97-94-9 -
 Triethylborane solution CAS 97-94-9View Details
Triethylborane solution CAS 97-94-9View Details
97-94-9 -
 Triethylborane solution CAS 97-94-9View Details
Triethylborane solution CAS 97-94-9View Details
97-94-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
