Borane-methyl sulfide complex
Synonym(s):(Dimethyl sulfide)trihydroboron;BMS;Borane-dimethyl sulfide;Trihydro[thiobis[methane]]boron
- CAS NO.:13292-87-0
- Empirical Formula: C2H10BS
- Molecular Weight: 76.97
- MDL number: MFCD00013189
- EINECS: 236-313-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
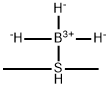
What is Borane-methyl sulfide complex?
Description
Borane dimethylsulfide (BMS) is a complex of borane with dimethylsulfide. The other common complex of borane is borane-THF. BMS is more stable than borane-THF and is therefore available in higher concentrations. BMS has the drawback of being smelly due to its dimethylsulfide content.
Chemical properties
clear colorless to light yellow solution
The Uses of Borane-methyl sulfide complex
Borane-dimethyl sulfide complex is used for hydroborations and reductions. Used with a dendrimeric supported L-pyrrolidinol in the asymmetric reduction of indanones and tetralones. CBS-catalyzed asymmetric reduction of ferrocenyl-1,3-diketones to 1,3-diols. Highly enantioselective reduction of ketones catalyzed by C3-symmetric tripodal hydroxyamides.
The Uses of Borane-methyl sulfide complex

To a solution of the SM (1.0 g, 6.4 mmol) in THF (18 mL) at 0 C was added BH3-SMe2 (6.4 mL, 12.8 mmol). The reaction mixture was stirred at 0 C for 2 h, then RT for 3 h. The mixture was cooled to 0 C and quenched with sat aq K2CO3 (5 mL). The layers were separated and the aq layer was extracted with EtOAc (2 x 15 mL) and diethyl ether (2 x 15 mL). The combined org layers were dried (Na2SO4) and concentrated to provide the product as a colorless oil. [1.0 g, 100 %]
The Uses of Borane-methyl sulfide complex
Borane-dimethyl sulfide (BH3 Me2S) can be used as a reagent:
- For the selective synthesis of 1,3,5-oxygenated compounds from dimethyl 3-oxoglutarate.
- For the conversion of ozonides to alcohols.
- In the CBS-catalyzed asymmetric reduction of ferrocenyl-1,3-diketones to 1,3-diols.
- For enantioselective reduction of ketones to chiral secondary alcohols in the presence of C3-symmetric tripodal hydroxyamide as a ligand.
- For the hydroboration reduction and other applications.
- With a dendrimeric supported L-pyrrolidinol in the asymmetric reduction of indanones and tetralones.
What are the applications of Application
Borane dimethyl sulfide complex is a convenient and stable source of borane
General Description
Borane dimethyl sulfide complex (BMS) acts as a highly efficient and selective reducing agent in the presence of catalytic sodium tetrahydroborate for α-hydroxy esters. Asymmetric borane reduction of a variety of prochiral ketones with BMS using spiroborate esters as catalyst has been reported.
Properties of Borane-methyl sulfide complex
| Melting point: | -40--37°C |
| Boiling point: | ~34 °C |
| Density | 1.287 g/mL at 25 °C |
| refractive index | 1.4570 |
| Flash point: | 65 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with ethyl ether, tetrahydrofurane, dichloromethane, benzene, xylene, hexane, diglyme, dimethyl ether, ethyl acetate, toluene, methylene chloride and other aprotic solvents. |
| appearance | Colorless liquid |
| form | Solution |
| color | Colorless to pale yellow |
| Specific Gravity | 0.801 |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| BRN | 3663489 |
| CAS DataBase Reference | 13292-87-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Borane-methyl sulfide complex(13292-87-0) |
| EPA Substance Registry System | Boron, trihydro[thiobis[methane]]-, (T-4)- (13292-87-0) |
Safety information for Borane-methyl sulfide complex
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H318:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Borane-methyl sulfide complex
| InChIKey | RMHDLBZYPISZOI-UHFFFAOYSA-N |
Borane-methyl sulfide complex manufacturer
JSK Chemicals
Sainor Laboratories Pvt Ltd Unit III
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Borane-dimethyl sulfide complex CAS 13292-87-0View Details
Borane-dimethyl sulfide complex CAS 13292-87-0View Details
13292-87-0 -
 Borane-dimethyl sulfide complex CAS 13292-87-0View Details
Borane-dimethyl sulfide complex CAS 13292-87-0View Details
13292-87-0 -
 Borane-dimethyl sulfide complex CAS 13292-87-0View Details
Borane-dimethyl sulfide complex CAS 13292-87-0View Details
13292-87-0 -
 Borane-dimethyl sulfide complex CAS 13292-87-0View Details
Borane-dimethyl sulfide complex CAS 13292-87-0View Details
13292-87-0 -
 Borane-dimethyl sulfide complex CAS 13292-87-0View Details
Borane-dimethyl sulfide complex CAS 13292-87-0View Details
13292-87-0 -
 Borane-dimethyl sulfide complex CAS 13292-87-0View Details
Borane-dimethyl sulfide complex CAS 13292-87-0View Details
13292-87-0 -
 Borane-dimethyl sulfide complex, 10 M in DMS 99%View Details
Borane-dimethyl sulfide complex, 10 M in DMS 99%View Details -
 Borane-dimethyl sulfide complex 99%View Details
Borane-dimethyl sulfide complex 99%View Details
