Triethylaluminum
Synonym(s):Aluminumtriethanide
- CAS NO.:97-93-8
- Empirical Formula: C6H15Al
- Molecular Weight: 114.16
- MDL number: MFCD00009015
- EINECS: 202-619-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-22 18:04:34
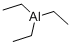
What is Triethylaluminum?
Chemical properties
Colorless liquid. Miscible with saturated hydrocarbons.
Chemical properties
The aluminum alkyls are highly flammable and reactive, colorless to yellow liquids at room temperature. The lighter trialkylaluminums ignite spontaneously in air. They are normally supplied and used in a 20% solution with a hydrocarbon solvent, such as hexane, heptane, benzene, toluene. Properties may depend on solvent. Reacts violently with water.
The Uses of Triethylaluminum
Triethylaluminum, in combination withmany transition metal complexes, is used as Ziegler-Natta polymerization and hydrogenationcatalyst. Also, it is used as intermediatein organic syntheses.
The Uses of Triethylaluminum
Catalyst intermediate for polymerization of olefins, especially ethylene; pyrophoric fuels; production of α-olefins and long-chain alcohols; gas plating of aluminum.
The Uses of Triethylaluminum
Triethylaluminum is used as a co-catalyst in the industrial production of polyethylene and for the production of medium chain alcohols. Used as a catalyst in Ziegler-Natta polymerization process for vinyl, olefin, diene polymerizations and linear oligomerization and cyclization of unsaturated hydrocarbons. It is also used as a catalyst to produce ethylene gas, chain growth of ethylene, longer chain aluminum alkyls, and in plating aluminum.
Production Methods
In a typical example, a slurry is prepared containing 7 wt % aluminum powder (0.1 wt% zirconium catalyst content) and 45 wt% recycled triethylaluminum in a paraffin hydrocarbon fraction, boiling range 177 – 260 ℃. The slurry is pumped into the reactor at a rate of 1.5 m3/h, at a reactor temperature of 132 ℃, while hydrogen is introduced at the bottom to maintain a partial pressure of 10 MPa. Excess hydrogen is removed in flash drum, wherein pressure is decreased to 3 MPa and temperature is lowered to prevent thermal reversal of the reaction. In the ethylation reactor sufficient ethylene is added to react completely with the Al – H bonds. Temperature is held at around 85 ℃ or somewhat higher. The addition rate increases with temperature, but carboalumination of ethylene also can occur to formn-butyl groups, especially at higher temperatures. Hydrogen and ethylene partial pressure is kept low in ethylation reactor to minimize hydrogenation of ethylene to ethane and to reduce excess carboalumination products. Excess ethylene and hydrogen are removed in flash drum. A portion of the triethylaluminum product solution is recycled to reactor.
General Description
A colorless liquid. Flammable gas is produced on contact with water.
Air & Water Reactions
Pyrophoric, ignites in moist air [Merck 11th ed. 1989]. Reacts violently with water.
Reactivity Profile
Triethylaluminum reacts violently with water, alcohols, phenols, amines, carbon dioxide, sulfur oxides, nitrogen oxides, halogens, and halogenated hydrocarbons, causing fire and explosion hazards. [Handling Chemicals Safely 1980 p. 929]. A mixture of dimethylformamide and triethyl aluminum exploded when heated [Bretherick 1995].
Health Hazard
Exposure to smoke from fire causes metal-fume fever (flu-like symptoms). Since liquid ignites spontaneously, contact with eyes or skin causes severe burns.
Health Hazard
The health hazard from exposure to thiscompound is attributed to its violent reactionswith many substances, including airand water. Because of its violent reactionwith moisture, skin contact can cause a dangerousburn. Contact with eyes can damagevision.
Fire Hazard
Triethylaluminum is extremely pyrophoric, igniting spontaneously in air. It reacts violently with water, alcohol, halogenated hydrocarbons, and oxidizing substances. Among the alcohols, the lower alcohols, methanol, ethanol, n- propanol, and isopropyl alcohol, react explosively with triethylaluminum. Reactions with lower aldehydes, ketones and amides can be vigorous to violent. It may explode on contact with halocarbons in excess molar ratios or upon slight warming. When heated to 200°C (392°F), it decomposes, liberating ethylene and hydrogen.
Flammability and Explosibility
Highly flammable
Safety Profile
Extremely destructive to living tissue. A very dangerous fire hazard when exposed to heat or flame. Ignites spontaneously in air. Explodes violently in water. To fight fire, use CO2, dry sand, dry chemical. Do not use water, foam, or halogenated fire-fighting agents. Explosive reaction with alcohols (e.g., methanol, ethanol, propanol), carbon tetrachloride, N,N-dmethylformamide + heat. Incompatible with halogenated hydrocarbons; triethyl borane. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALUMINUM COMPOUNDS and ORGANOMETALS.
Potential Exposure
Alkyl aluminum compounds are used as components of olefin polymerization catalysts. They are also used in the synthesis of higher primary alcohols and in pyrophoric fuels, as a catalyst in making ethylene gas; and in plating aluminum.
Shipping
ntial fire or explosion hazard. Shipping: UN3399 Organometallic substance, liquid, water-reactive, flammable, Hazard Class: 4.3; Labels: 4.3 Dangerous Dangerous when wet material, 3-Flammable liquid, technical name Required. UN3051-Spontaneously combustible. Also, this material is dangerous when wet. (Note: this number does not appear in the 49/CFR HazMat tables).
Purification Methods
Purify it by fractionation in an inert atmosphere under a vacuum in a 50cm column containing a heated nichrome spiral, taking the fraction b 112-114o/27mm. It is very sensitive to H2O and should be stored under N2. It should not contain chloride ions which can be shown by hydrolysis and testing with AgNO3. [Baker & Sisler J Am Chem Soc 75 4828 5193 1953, NMR: Brownstein et al. J Am Chem Soc 81 3826 1959, Beilstein 4 IV 4398.]
Incompatibilities
The lighter trialkylaluminums ignite spontaneously in air; can self-heat in the air at room temperature without any added energy and may ignite. These compounds are strong reducing agents. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with water, oxygen (air), acids, alcohols, phenols, amines, carbon dioxide; sulfur oxides; halogenated compounds, and many other substances
Waste Disposal
Careful incineration
Properties of Triethylaluminum
| Melting point: | -50°C |
| Boiling point: | 128-130°C (50 mmHg) |
| Density | 0.85 g/mL at 20 °C |
| vapor pressure | 1 mmHg ( 62.2 °C) |
| Flash point: | −1 °F |
| storage temp. | 0-6°C
|
| form | liquid |
| color | colorless |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| BRN | 3587229 |
| Exposure limits | ACGIH: TWA 50 ppm (Skin) OSHA: TWA 500 ppm(1800 mg/m3) NIOSH: IDLH 1100 ppm; TWA 50 ppm(180 mg/m3) |
| Dielectric constant | 6.4(20℃) |
| CAS DataBase Reference | 97-93-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Triethylaluminum(97-93-8) |
| EPA Substance Registry System | Triethylaluminum (97-93-8) |
Safety information for Triethylaluminum
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H250:Pyrophoric liquids; Pyrorophoric solids H252:Self-heating substances and mixtures H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H304:Aspiration hazard H314:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Triethylaluminum
| InChIKey | VOITXYVAKOUIBA-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![hydrogen 5-methoxy-2-[[5-methoxy-3-(3-sulphonatopropyl)-3H-benzothiazol-2-ylidene]methyl]-3-(3-sulphonatopropyl)benzothiazolium, compound with triethylamine (1:1)](https://img.chemicalbook.in/CAS/GIF/63148-97-0.gif)
You may like
-
 Triethylaluminium 1.8M in Hexanes CAS 97-93-8View Details
Triethylaluminium 1.8M in Hexanes CAS 97-93-8View Details
97-93-8 -
 Triethylaluminium 1M In Hexanes CAS 97-93-8View Details
Triethylaluminium 1M In Hexanes CAS 97-93-8View Details
97-93-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1