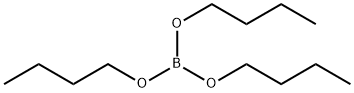
Triethyl borate
Synonym(s):Boric acid triethyl ester;Boron ethoxide;Triethoxyborane
- CAS NO.:150-46-9
- Empirical Formula: C6H15BO3
- Molecular Weight: 145.99
- MDL number: MFCD00009073
- EINECS: 205-760-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Triethyl borate?
Chemical properties
Triethyl borate is a Colorless liquid; mild odor. Hydrolyzes rapidly depositing boric acid in finely divided crystalline form.
The Uses of Triethyl borate
Antiseptics, disinfectants, antiknock agent.
Triethyl borate is used as a solvent in resins, paints, varnishes, adhesives and sealant chemicals. It is used as a catalyst of synthetic waxes and resins. It is an active component of flame retardants in the textile industry and in welding fluxes. It is used to prepare m-tolyl-acetic acid ethyl ester by reacting with 1-bromomethyl-3-methyl-benzene and carbon monoxide using as 1,5-hexadiene dium chloride as a catalyst. Further, it is used in pyrotechnics.
Triethyl borate may be used as boron source to synthesize lithium borophosphates, alumina borosilicate ceramic nanofibres and boron-doped single wall carbon nanotubes.CVD precursor for boron modified SiO2 in microelectronics Intermediate for organic-modified borosiloxanes.
The Uses of Triethyl borate
Triethyl borate is used as a solvent in resins, paints, varnishes, adhesives and sealant chemicals. It is used as a catalyst of synthetic waxes and resins. It is an active component of flame retardants in the textile industry and in welding fluxes. It is used to prepare m-tolyl-acetic acid ethyl ester by reacting with 1-bromomethyl-3-methyl-benzene and carbon monoxide using as1,5-hexadiene?dium chloride as a catalyst. Further, it is used in pyrotechnics.
Definition
ChEBI: A member of the class of borate esters obtained by the formal condensation of three equivalents of ethanol with boric acid.
Preparation
Triethyl borate can be prepared by adding anhydrous ethanol with boric acid or boron trioxide. Can be purified via distillation.
Hazard
Flammable, dangerous fire risk.
Safety Profile
Moderately toxic by ingestion. Aneye irritant. A very dangerous fire hazard when exposed toheat or flame. When heated to decomposition it emitsacrid smoke and irritating fumes.
Purification Methods
Dry it over sodium, then distil it. Also fractionate it through a gauze packed column. [Charnlet et al. J Chem Soc 2288 1952, as for tributyl borate Johnson & Tompkins Org Synth Coll Vol II 106 1943, Beilstein 1 III 1339, 1 IV 1365.]
Properties of Triethyl borate
| Melting point: | -84.5 °C |
| Boiling point: | 117-118 °C(lit.) |
| Density | 0.858 g/mL at 25 °C(lit.) |
| vapor density | 5.04 (vs air) |
| refractive index | n |
| Flash point: | 52 °F |
| storage temp. | 0-6°C
|
| solubility | Miscible with alcohol and ether. |
| form | Liquid |
| Specific Gravity | 0.864 |
| color | Colorless |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1699052 |
| CAS DataBase Reference | 150-46-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Boric acid, triethyl ester(150-46-9) |
| EPA Substance Registry System | Boric acid (H3BO3), triethyl ester (150-46-9) |
Safety information for Triethyl borate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triethyl borate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 150-46-9 TRIETHYL BORATE 99%View Details
150-46-9 TRIETHYL BORATE 99%View Details
150-46-9 -
 Triethyl borate (TEB)View Details
Triethyl borate (TEB)View Details
150-46-9 -
 Triethyl Borate CAS 150-46-9View Details
Triethyl Borate CAS 150-46-9View Details
150-46-9 -
 Triethyl borate 98% (GC) CAS 150-46-9View Details
Triethyl borate 98% (GC) CAS 150-46-9View Details
150-46-9 -
 Triethyl borate CAS 150-46-9View Details
Triethyl borate CAS 150-46-9View Details
150-46-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
