triclofos
- CAS NO.:306-52-5
- Empirical Formula: C2H4Cl3O4P
- Molecular Weight: 229.38
- MDL number: MFCD00242992
- EINECS: 206-185-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
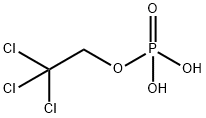
What is triclofos?
Chemical properties
White powder with saline taste; soluble in water, almost insoluble in ether, and slightly soluble in alcohol (1 g/ 250 mL). Hygroscopic in air. Stable in light; unstable in above ambient temperatures.
Originator
Triclos,Merrell National,US,1972
The Uses of triclofos
Triclofos is a hypnotic and sedative recommended for insomnia, nocturnal awakening, and early morning awakening.
Production Methods
Chloral is reduced with sodium borohydride and the resulting trichloroethanol is esterified with polyphosphoric acid to give the dihydrogen phosphate. The ester is then reacted with an equimolar quantity of sodium hydroxide.
Definition
ChEBI: Triclofos is a monoalkyl phosphate.
Manufacturing Process
Trichloroethanol (500 grams) and phosphorus oxychloride (510 grams) were
added to dry diethyl ether (3.5 liters) and stirred at 10°C with ice/water
cooling. Dry pyridine (270 ml) was added dropwise over 1 hour, maintaining
the temperature below 25°C. The resulting suspension was stirred for a
further 1 hour and then stood at 0°C overnight. The pyridine hydrochloride
was removed by filtration and washed with diethyl ether (2 x 300 ml) and
dried in vacuo over P2O5 to give 380 grams.
The ether filtrate and washings were evaporated at room temperature under
reduced pressure to give a clear liquid residue (801 grams). This residue was
distilled under high vacuum to give trichloroethyl phosphorodichloridate (556
grams, 62.4% of theory), boiling point 75°C/0.8 mm.
The phosphorodichloridate was hydrolyzed by adding to a stirred solution of
sodium carbonate (253 grams) in water (2.9 liters). After 1 hour the solution
was cooled and acidified with a solution of concentrated sulfuric acid (30 ml)
in water (150 ml) and then extracted with a mixture of tetrahydrofuran and
chloroform (2.3/1; 3 x 1 liter). The tetrahydrofuran/chloroform liquors were
bulked and evaporated to dryness to give a light brown oil. This was dissolved
in water (1 liter) and titrated with 2 N sodium hydroxide solution to a pH of
4.05 (volume required 930 ml). The aqueous solution was clarified by filtration
through kieselguhr and then evaporated under reduced pressure to a syrup
(737 grams).
Hot acetone (4.5 liters) was added to this syrup and the clear solution stood
at room temperature for 2 hours and then at 0°C overnight. The white
crystalline solid was filtered off, washed with acetone (2 x 400 ml) and dried
at 60°C in vacuo to give sodium trichloroethyl hydrogen phosphate (414
grams, 49.3% of theory from trichloroethanol).
Therapeutic Function
Sedative, Hypnotic
Safety Profile
Moderately toxic by ingestion.When heated to decomposition it emits toxic vapors ofPOx and Clí.
Metabolism
Not Available
Properties of triclofos
| Boiling point: | 321.2±52.0 °C(Predicted) |
| Density | 1.891±0.06 g/cm3(Predicted) |
| pka | 1.65±0.10(Predicted) |
| EPA Substance Registry System | Ethanol, 2,2,2-trichloro-, dihydrogen phosphate (306-52-5) |
Safety information for triclofos
Computed Descriptors for triclofos
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4