Triadimefon
Synonym(s):1-(4-Chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)-2-butanone
- CAS NO.:43121-43-3
- Empirical Formula: C14H16ClN3O2
- Molecular Weight: 293.75
- MDL number: MFCD00055506
- EINECS: 256-103-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-07 19:09:50
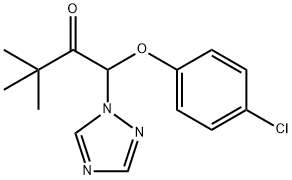
What is Triadimefon?
Description
Triadimefon has been a widely used fungicide on crops and nonfood products since the early 1970s. The metabolite triadimenol is also active and is registered separately for use as seed treatment. Triadimenol has a broad regulatory toxicology database, but its toxicity is considered to be encompassed in that of triadimefon and therefore the same study was used by the United States Environmental Protection Agency (US EPA) in establishing regulatory levels for both pesticides. In nontarget species, dopaminergic neurotoxicity is the primary effect, but with chronic exposures its toxicities include hepatic, carcinogenic, developmental, and reproductive effects.
The Uses of Triadimefon
Triadimefon is used for the control of powdery mildews in cereals, pome fruit, stone fruit, berry fruit, vines, hops, cucurbits, tomatoes, vegetables, sugar beet, mangoes, ornamentals, turf, flowers, shrubs and trees, Monilinia spp. in stone fruit, black rot of grapes, leaf blotch, leaf spot and snow mould in cereals, pineapple disease butt rot in pineapples and sugar cane, leaf spots and flower blight in flowers, shrubs and trees and many other diseases of turf.
The Uses of Triadimefon
antifungal, P450 inhibitor
The Uses of Triadimefon
Triadimefon is an triazole fungicide is used for the management of mango powdery mildew in South Gujarat.
The Uses of Triadimefon
Systemic agricultural fungicide.
The Uses of Triadimefon
Systemic fungicide used to control mildews and rusts that attack coffee, cereals, stone fruit, grapes and ornamentals.
What are the applications of Application
Triadimefon is an antifungal cytochrome P450 inhibitor
Definition
ChEBI: 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one is a member of the class of triazoles that is 1-hydroxy-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one in which the hydroxyl hydrogen is replaced by a 4-chlorophenyl group. It is a member of triazoles, a member of monochlorobenzenes, an aromatic ether, a ketone and a hemiaminal ether.
General Description
Colorless to pale yellow crystalline solid with a slight odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Triadimefon is incompatible with strong oxidizing agents and acids. Reacts with acid halides and anhydrides. Also reacts with most active hydrogen compounds .
Fire Hazard
Flash point data for Triadimefon are not available; however, Triadimefon is probably combustible.
Agricultural Uses
Fungicide: Triadimefon is a systemic fungicide that is used to control powdery mildews, rusts, and other fungi on coffee, seed grasses, cereals, fruits, grapes, vegetables, vines, pineapple, sugar cane, sugar beets, turf, shrubs, and trees. Not approved for use in EU countries. Registered for use in the U.S. U.S. Maximum Allowable Residue Levels for Triadimefon
Trade name
ACCOST®; ACIZOL®; AMIRAL®; BAY® 6681-F; BAYLETON®; BAY®-MEB-6447; BAYER® 6681-F; BAYER® MEB-6447; MEB 6447®; PRO-TEK®; ROFON®
Pharmacology
Triadimefon (36) and its alcohol analog triadimenol (37) have been intensively investigated to determine the influence of their enantiomeric difference on fungicidal activity. Between stereoisomeric triadimefon, no significant difference is observed in their fungicidal activity. However, triadimenol, which shows a much higher fungicidal activity than triadimefon, exhibits a clear stereochemistry-dependent activity difference. Greater fungicidal activity is possessed by the (1S, 2R)-isomer (28).
Safety Profile
Poison by ingestion. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cland NOx. See also KETONES.
Environmental Fate
Soil. In a culture study, the microorganism Aspergillus niger degraded 32% of tri-
adimefon to triadimenol after 5 days (Clark et al., 1978).
Plant. In soils and plants, triadimefon degrades to triadimenol (Clark et al., 1978;
Rouchaud et al., 1981). In barley plants, triadimefon was metabolized to triadimenol and
p-chlorophenol (Rouchaud et al., 1981; Rouchaud, 1982). In the grains an
Photolytic. When triadimefon was subjected to UV light for one week, p-chlorophenol,
4-chlorophenyl methyl carbamate and a 1,2,4-triazole formed as products (Clark et al.,
1978).
Metabolic pathway
Enzymic reduction of triadimefon is an important pathway in plants, soils and fungi and may be regarded as an activation process, which produces fungicidally active triadimenol. Two diastereoisomers of triadimenol, A and B [( 1RS,2SR)-l-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl) butan-2-ol is referred to as diastereoisomer A; 1RS,2RS- is referred to as diastereoisomer β], are produced in different amounts by plants and fungi and the proportions may differ within the plant. Similar metabolic pathways are followed in mammals where reduction of the keto group yields triadimenol as the principal metabolite and oxidation of the butyl group gives alcohol and carboxylic acid derivatives.
Degradation
Triadimefon is stable to hydrolysis with a DT50 of more than 1 year at pH
3,6 and 9 (22 °C).
On photolysis in methanol in borosilicate glass apparatus using a
medium pressure mercury lamp, triadimefon undergoes cleavage of the
C-1-N bond giving 1,2,4-triazole (2), 4-chlorophenyl methyl carbonate
(3) and 4-chlorophenol(4) (Clark et al., 1978) (Scheme 1).
Sensitised photolysis of triadimefon irradiated by light from a highpressure
mercury lamp, with a Pyrex filter to exclude wavelengths below
290 nm, in the presence of fulvic acid and humic acid gave a variety of
products. In water, the products formed were 4 and a dihydroxychlorobenzene
(5). Although there are some ambiguities in the report concerning
the allocation of structures to the compounds obtained, these included a dihydroxybenzaldehyde (6) and 5-chlorosalicylaldehyde (7). Major
products in the presence of fulvic acid were 4 and a dihydroxychlorobenzene
(5). In the presence of humic acid 4,5, a dihydroxybenzaldehyde
(6) and 1-phenoxy-33-dimethyl-1- ( 1H-1,2,4-triazol-l- yl) -2-butanone (8)
were formed (Moza et al., 1995).
Toxicity evaluation
Triadimefon inhibits the lanosterol demethylase, thereby
interfering with ergosterol synthesis that is necessary for the
integrity of fungal cell walls. This action confers specificity for
fungi over vertebrates; however, by a similar mechanism
triazoles have been reported to disrupt steroid and cholesterol
metabolism in mammals. Perturbations of fatty acid, steroid,
and xenobiotic metabolism pathways in liver through specific
nuclear signaling pathways (constitutive androstane receptor
(CAR) and pregnane X receptor (PXR)) have been suggested to
contribute to the observed reproductive and hepatic toxicities.
Triadimefon also both inhibits and induces specific hepatic
cytochrome P-450 enzymes. A series of studies comparing
triadimefon with other two conazoles (propiconazole and
myclobutanil) have shown different modes of action in terms
of carcinogenicity, hepatotoxicity, and developmental and
reproductive toxicities.
Studies in several species have shown that neurotoxicity is the
endpoint of concern with both acute and repeated exposures to
triadimefon and triadimenol. Triadimefon causes accumulation
of synaptic dopamine, both in vivo and in vitro. Pharmacological
challenges and neurochemical studies have shown that
triadimefon blocks dopamine reuptake by binding to the
dopamine transporter in a manner similar to other indirect
dopamine agonists, such as cocaine and d-amphetamine.
Properties of Triadimefon
| Melting point: | 82°C |
| Boiling point: | 441.9±55.0 °C(Predicted) |
| Density | 1.2200 |
| vapor pressure | 2 x l0-5 Pa (20 °C) |
| refractive index | 1.5388 (estimate) |
| Flash point: | 11 °C |
| storage temp. | APPROX 4°C
|
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; DMSO:PBS(pH7.2) (1:1): 0.5 mg/ml; Ethanol: 10 mg/ml |
| form | Solid |
| pka | 1.41±0.11(Predicted) |
| form | neat |
| color | White to off-white |
| Water Solubility | 0.026 g/100 mL |
| Merck | 13,9666 |
| BRN | 619231 |
| NIST Chemistry Reference | 2-Butanone, 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1h-1,2,4-triazol-1-yl)-(43121-43-3) |
| EPA Substance Registry System | Triadimefon (43121-43-3) |
Safety information for Triadimefon
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H330:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Triadimefon
Triadimefon manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Triadimefon CAS 43121-43-3View Details
Triadimefon CAS 43121-43-3View Details
43121-43-3 -
 Triadimefon CAS 43121-43-3View Details
Triadimefon CAS 43121-43-3View Details
43121-43-3 -
 Triadimefon 25 WpView Details
Triadimefon 25 WpView Details
43121-43-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
