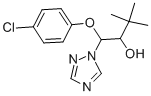
Triadimenol
Synonym(s):α-tert-Butyl-β-(4-chlorophenoxy)-1H-1,2,4-triazole-1-ethanol
- CAS NO.:55219-65-3
- Empirical Formula: C14H18ClN3O2
- Molecular Weight: 295.76
- MDL number: MFCD00055507
- EINECS: 259-537-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is Triadimenol?
Description
Triadimenol is a metabolite of triadimefon , a broad-spectrum chiral triazole fungicide, that is formed by reduction of a carbonyl group to the corresponding alcohol. It is teratogenic, inducing cranial nerve and ganglia abnormalities in a rat post-implantation whole embryo culture model when used at concentrations ranging from 12.5 to 125 μM. In vivo, triadimenol induces embryotoxicity in rats and rabbits when administered orally at doses of 100 and 40 mg/kg, respectively. Embryonic exposure to triadimenol (3-3,000 μg/L) induces embryonic mortality as well as decreases fertility and increases the number of female offspring in medaka fish (O. latipes).
The Uses of Triadimenol
Triadimenol is used for the control of powdery mildews, rusts and Rhynchosporium in cereals, and also as a seed treatment to control bunt, smut and other cereal diseases. It is also used on vines, hops, coffee, tomatoes, vegetables, fruit, tobacco, sugar cane, ornamentals and other crops, mainly against powdery mildews, rusts and various leaf spot diseases.
The Uses of Triadimenol
β-(4-Chlorophenoxy)-α-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol is a agricultural fungicide that is systemically active against powdery mildews and rusts of grains.
The Uses of Triadimenol
Systemic agricultural fungicide; cereal seed protectant.
What are the applications of Application
Triadimenol is a triazole-type anti-fungal demonstrating estrogen receptor agonism
Definition
ChEBI: A member of the class of triazoles that is 3,3-dimethyl-1-(1,2,4-triazol-1-yl)butane-1,2-diol substituted at position O1 by a 4-chlorophenyl group. A fungicide for cereals, beet and brassicas used to control a range of diseases including powdery mildew, ru ts, bunts and smuts.
Agricultural Uses
Fungicide: Triadimenol is used to control seed-and soil-borne diseases and to provide early season control of foliar diseases. It is applied to seeds of barley, corn, oats, rye, sorghum and wheat and also to fruits, vegetables and ornamentals. Registered for use in EU countries . Registered for use in the U.S. U.S. Maximum Allowable Residue Levels for Triadimenol
Trade name
BAYFIDAN®; BAYFRDAN EW®; BAY KWG 0519®; BAYTAN® SEED TREATMENT; BAYTAN 30® FUNGICIDE; PROTEGE ALLEGIANCE BAYTAN®; SPINNAKER®; SUMMIT®; TRIADIMENOL®; TRIAFOL®; TRIAPHOL®
Metabolic pathway
When triadimenol is irradiated by UV light in methanol solution, two major degradation products are identified as 1-(4-chlorophenoxy)-3,3-dimethylbutan-2-one and 1-phenoxy-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan- 2-ol. When a spray deposit on apple leaves is exposed outdoors to natural sunlight, the only product detected is the butanone metabolite. The butanone metabolite has very low fungicidal activity against apple powdery mildew, whereas the butanol metabolite gives some control of infection.
Degradation
When a methanolic solution of triadimenol (1) in borosilicate glass apparatus was irradiated by a medium pressure mercury lamp for 20 days, 50% of the triadimenol was photodegraded. The products identified were 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H -1,2,4-triazol-1-yl)butan-2- one (major product) (2), 4-chlorophenol (3) and 1-phenoxy-3,3-dimethyl- 1-(1H-1,2,4-triazol-1-yl)butan-2-ol(4 ). When triadimenol was irradiated as a solid deposit, 55% of the parent compound was recovered after 18 days and the same products were identified. Losses may be accounted for by volatilisation or photodegradation (Clark and Watkins, 1986) (Scheme 1). The only major product detected 33 days after triadimenol had been applied to apple tree leaf surfaces exposed to light outdoors was 2.
Properties of Triadimenol
| Melting point: | 112-117° |
| Boiling point: | 465.4±55.0 °C(Predicted) |
| Density | 1.2990 (rough estimate) |
| vapor pressure | A 6 x l0-7 Pa (20 °C); B 4 x l0-7 Pa (20 °C) |
| refractive index | 1.5270 (estimate) |
| Flash point: | 2 °C |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| Water Solubility | A 62 mg l-1 (20 °C); B 33 mg l-1(20 °C) |
| pka | 13.29±0.20(Predicted) |
| color | White to Off-White |
| Merck | 13,9667 |
| BRN | 616470 |
| CAS DataBase Reference | 55219-65-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Triadimenol(55219-65-3) |
| EPA Substance Registry System | Triadimenol (55219-65-3) |
Safety information for Triadimenol
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H362:Reproductive toxicity, effects on or via lactation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Triadimenol
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
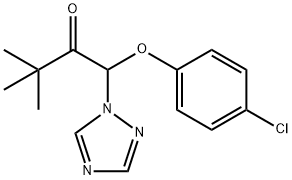
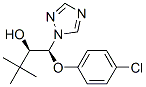

You may like
-
 Triadimenol CAS 55219-65-3View Details
Triadimenol CAS 55219-65-3View Details
55219-65-3 -
 Triadimenol CAS 55219-65-3View Details
Triadimenol CAS 55219-65-3View Details
55219-65-3 -
 Triadimenol CAS 55219-65-3View Details
Triadimenol CAS 55219-65-3View Details
55219-65-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1