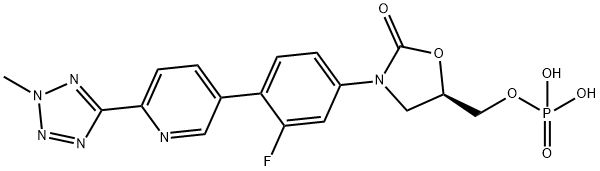
Torezolid
- CAS NO.:856866-72-3
- Empirical Formula: C17H15FN6O3
- Molecular Weight: 370.34
- MDL number: MFCD19442562
- EINECS: 815-370-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-16 20:45:37

What is Torezolid?
Absorption
Tedizolid reaches peak plasma concentrations within three hours for oral administration and within one hour following intravenous administration; the absolute oral bioavailability is approximately 91%. Food has no effect on absorption. When given once daily, either orally or intravenously, tedizolid reaches steady-state concentrations in approximately three days.
The Cmax for tedizolid after a single dose/at steady-state is 2.0 ± 0.7/2.2 ± 0.6 mcg/mL for oral administration, and 2.3 ± 0.6/3.0 ± 0.7 mcg/mL for intravenous administration, respectively. Similarly, the Tmax has a median (range) of 2.5 (1.0 - 8.0)/3.5 (1.0 - 6.0) hrs for the oral route and 1.1 (0.9 - 1.5)/1.2 (0.9 - 1.5) hrs when given intravenous. The AUC is 23.8 ± 6.8/25.6 ± 8.4 mcg*hr/mL for oral and 26.6 ± 5.2/29.2 ± 6.2 mcg*hr/mL for intravenous.
Toxicity
Toxicity information regarding tedizolid is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as nausea, headache, dizziness, diarrhea, and vomiting. Symptomatic and supportive measures are recommended.
The Uses of Torezolid
Tedizolid, known as TR-700, is an oral and i.v administered intracellular antibacterial drug.
Background
Drug-resistant bacteria, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and penicillin-resistant Streptococcus penumoniae, represent a massive public health threat. Tedizolid is a member of the oxazolidinone class of antibiotics, which includes the previously approved linezolid and is generally effective against multidrug-resistant Gram-positive bacteria. Tedizolid is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and is generally more effective and more tolerable than linezolid.
Tedizolid was approved by the FDA on June 20, 2014, for sale by Cubist Pharmaceuticals as tedizolid phosphate (SIVEXTRO?). This product is currently available as both an oral tablet and as a powder for intravenous injection.
Indications
Tedizolid is indicated for the treatment of acute bacterial infections of the skin and skin structure (ABSSSI). To prevent drug resistance, tedizolid should only be used for infections that are caused by susceptible bacteria.
What are the applications of Application
Tedizolid is an antibacterial
Definition
ChEBI: A member of the class of pyridines that is pyridine which is substituted by a 2-methyl-2H-tetrazol-5-yl group at position 2 and by a 2-fluoro-4-[(5R)-5-(hydroxymethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl group at position 5 It is used as its phosphate pro-drug used for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and meth cillin-susceptible strains), various Streptococcus species, and Enterococcus faecalis.
Biological Activity
tedizolid is an oxazolidinone antimicrobial agent with mic50 value of 0.5 μg/ml for mssa, mrsa, vr e. faecium and vr e. faecalis and 0.25 μg/ml for msse, mrse, pssp and prsp [1].the resistant gram-positive infection is a serious global health problem. for instant, the methicillin-resistant s. aureus (mrsa) has spread all over the world with rates ranging from 18 to 26 cases among 100,000 people. besides that, there come out a serious of resistant strains such as the linezolid-resistant s. aureus (lrsa) and the vancomycin-resistant s. aureus (vrsa). due to the unfavorable outcomes of the existed antibiotics, alternative treatments have been developed. tedizolid is a synthetic antibiotic that works based on the inhibition of protein synthesis. it binds to the 50s ribosome and inhibits the formation of the 70s complex [1].tedizolid showed potent bacteriostatic activity against many resistant gram-positive pathogens such as mssa, mrsa, s. pyogenes and s. pneumoniae. for the enterococcal and staphylococcal isolates, tedizolid displayed more than 4-fold higher potency than that of linezolid. it also showed inhibitory effects on a panel of 169 linezolid-resistant staphylococcal isolates with 79.2% inhibition at concentration of ≤ 4μg/ml. the mic values of tedizolid against linezolid-resistant staphylococci were in a range from 0.06 to 16 mg/l. besides that, tedizolid was found to be the inhibitors of human monoamine oxidase with ic50 values of 8.7 and 5.7 μm for mao-a and mao-b, respectively [1, 2 and 3].when treated in vivo, tedizolid was the active moiety converted from the pro-drug tedizolid phosphate. it was found that granulocytes could affect the antistaphylococcal effect of tedizolid. in neutropenic mice, the administration of tedizolid for 24 hours or 48 hours caused ed50 values of 25.2 and 35.7 mg/kg/day, respectively [1 and 4].
Pharmacokinetics
Tedizolid is an oxazolidinone antibiotic that works by inhibiting protein synthesis by bacterial ribosomes. However, oxazolidinone antibiotics can also bind to human mitochondrial, but not cytoplasmic, ribosomes. Mitochondrial protein synthesis inhibition is associated with adverse patient effects such as neurological, hematological, and gastrointestinal toxicity, although tedizolid is tolerated better than the related linezolid. Alternative therapies should be considered when treating neutropenic patients with ABSSSI. Clostridium difficile-associated diarrhea has been reported in patients treated with tedizolid.
Metabolism
Tedizolid is administered as a phosphate prodrug that is converted to tedizolid (the circulating active moiety). Prior to excretion, the majority of tedizolid is converted to an inactive sulphate conjugate in the liver, though this is unlikely to involve the action of cytochrome P450-family enzymes.
References
[1] kanafani z a, corey g r. tedizolid (tr-701): a new oxazolidinone with enhanced potency. expert opinion on investigational drugs, 2012, 21(4): 515-522.
[2] rodríguez-avial i, culebras e, betriu c, et al. in vitro activity of tedizolid (tr-700) against linezolid-resistant staphylococci. journal of antimicrobial chemotherapy, 2012, 67(1): 167-169.
[3] flanagan s, bartizal k, minassian s l, et al. in vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. antimicrobial agents and chemotherapy, 2013, 57(7): 3060-3066.
[4] louie a, liu w, kulawy r, et al. in vivo pharmacodynamics of torezolid phosphate (tr-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant staphylococcus aureus strains in a mouse thigh infection model. antimicrobial agents and chemotherapy, 2011, 55(7): 3453-3460.
Properties of Torezolid
| Melting point: | 201 °C |
| Boiling point: | 614.5±65.0 °C(Predicted) |
| Density | 1.57 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Water (Slightly, Heated) |
| form | Solid |
| pka | 14.05±0.10(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C17H15FN6O3/c1-23-21-16(20-22-23)15-5-2-10(7-19-15)13-4-3-11(6-14(13)18)24-8-12(9-25)27-17(24)26/h2-7,12,25H,8-9H2,1H3/t12-/m1/s1 |
| CAS DataBase Reference | 856866-72-3 |
Safety information for Torezolid
Computed Descriptors for Torezolid
| InChIKey | XFALPSLJIHVRKE-GFCCVEGCSA-N |
| SMILES | O1[C@@H](CO)CN(C2=CC=C(C3=CC=C(C4=NN(C)N=N4)N=C3)C(F)=C2)C1=O |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
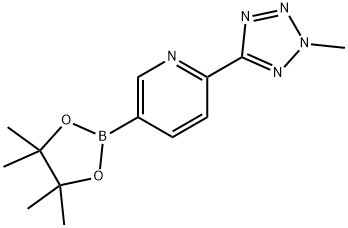
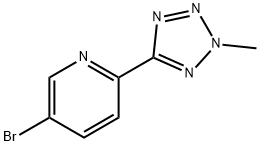
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](https://img.chemicalbook.in/CAS/GIF/1220910-89-3.gif)
You may like
-
 856866-72-3 Tedizolid 99%View Details
856866-72-3 Tedizolid 99%View Details
856866-72-3 -
 856866-72-3 98%View Details
856866-72-3 98%View Details
856866-72-3 -
 Torezolid 98% (HPLC) CAS 856866-72-3View Details
Torezolid 98% (HPLC) CAS 856866-72-3View Details
856866-72-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
