Topotecan
Synonym(s):9-[(dimethylamino)methyl]-10-hydroxy-(20S)-camptothecin hydrochloride hydrate;hycamptamine hydrochloride hydrate;Hydrogen chloride solution;NSC-609669 hydrochloride hydrate;SKF-104864A hydrochloride hydrate
- CAS NO.:123948-87-8
- Empirical Formula: C23H23N3O5
- Molecular Weight: 421.45
- MDL number: MFCD00870670
- EINECS: 687-471-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
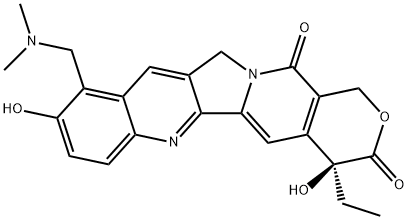
What is Topotecan?
Toxicity
The primary anticipated complication of overdosage would consist of bone marrow suppression.
Description
Topotecan was launched in the US for the second-line treatment of ovarian cancer. It can be prepared in four steps from camptothecin and is a water soluble derivative of the natural product with decreased toxicity. Unlike its chemical relative irinotecan, topotecacan in not a prodrug and does not require bioactivation. It is an inhibitor of topoisomerase I. Specifically, it inhibits the release of topoisomerase I from DNA, where it relaxes supercoiled DNA, giving rise to single-strand breaks. When the replication fork reaches this complex, double-stand breaks can occur. This signals apoptosis and eventually gives rise to cell death. Evidence indicates hycamtin is safe for people with impaired hepatic function.
Chemical properties
Off-white Cryst
Originator
SmithKline Beecham (UK)
The Uses of Topotecan
(S)-Topotecan is a DNA topoisomerase I inhibitor; semisynthetic analog of Camptothecin. Antineoplastic. Topotecan hydrochloride is a chemotherapy agent that is a topoisomerase 1 inhibitor.
The Uses of Topotecan
antineoplastic;DNA topoisomerase type 1 inhibitor
The Uses of Topotecan
Topotecan has been used as a positive control for the identification and analysis of HIF-1α and VEGF inhibitors in human glioma cells under hypoxic conditions1. It has also been used for in vitro apoptosis assays in PA317 cells2.
Background
An antineoplastic agent used to treat ovarian cancer. It works by inhibiting DNA topoisomerases, type I.
Indications
For the treatment of advanced ovarian cancer in patients with disease that has recurred or progressed following therapy with platinum-based regimens. Also used as a second-line therapy for treatment-sensitive small cell lung cancer, as well as in combination with cisplatin for the treatment of stage IV-B, recurrent, or persistent cervical cancer not amenable to curative treatment with surgery and/or radiation therapy.
What are the applications of Application
Topotecan is a topo I inhibitor shown to stabilize topo I/DNA cleavable complexes
Definition
ChEBI: A pyranoindolizinoquinoline used as an antineoplastic agent. It is a derivative of camptothecin and works by binding to the topoisomerase I-DNA complex and preventing religation of these 328 single strand breaks.
Manufacturing Process
Camptothecin (CPT) - a compound isolated from the bark, leaves and fruit of
Camptotheca acuminate (Wall M. E. et al., J. Am. Chem. Soc. 88, 3888,
1966).
10-Hydroxycamptothecin (10-HCPT) was prepared by subjecting CPT (3.2 g
0.0092 mol), 0.8 g of Pt0 (prepared by pre-reduction of 8 g of amorphous
PtO2 in 80 ml of acetic acid for 1.5 h under 1 atm hydrogen pressure) and
acetic acid to 1 atm of H2 for 8.5 h after which theoretical amount of H2
absorbed (slightly more than 0.4 L) and uptake of H2 gets slowed down. The
reaction mixture was degassed under steam of helium and filtered through
celite and washed with acetic acid (20 ml). The resulting solution was treated
immediately with Pb(OAc)4 (6.4 g 0.014 mol) in portions and reaction
mixture, stirred vigorously under helium for 30 min. Gumy residue was
obtained on evaporation of solvent which was triturated with cold water (100
ml) to produce light brown solid. The solid was collected, washed with cold
water and air dried overnight when a mixture of 10-HCPT (44%), acetyl 10-
hydroxycamptothecin (10-AcHCPT, 26%) and unreacted CPT (32%) on HPLC
basis was obtained. This crude mixture was combined with 150 ml of 50%
acetic acid and heated under reflux conditions overnight. The reaction mixture
was cooled, concentrated to 20 ml and treated with cold water (100 ml) to
produce precipitate, which is filtered, washed with more cold water and dried
to afford 2.1 g of solid containing 10-HCPT (70%), 10-AcCPT (1.2%) and CPT
(21.3%) on the basis HPLC. Mixture was triturating with 0.5% aq HCl to
dissolve the water-soluble. When insoluble CPT was removed by filtration.
Water-soluble was extracted with chloroform and crystallized from boiling
solution of 20% of MeOH in CHCl3 by adding EtOAC dropwise until turbidity
appeared to obtain pure yellow 10-(HCPT), melting point 268°-270°C.
10-HCPT (0.364 g 0.01 mmol) and 40% aqueous dimethylamine (12 ml) was
added in dichloromethane (50 ml) in which anhydrous potassium carbonate
(2.17 g, 15 mmol) has been suspended. The reaction mixture was stirred at
room temperature for 5 h, then filtered and solid extracted with ethylacetate
(20 ml). The solvent is evaporated in vacuo giving a residue. The residue was
triturated with 0.5% aq HCl (50 ml) to dissolve the water-soluble adduct.
Water-soluble were partitioned with petroleum ether (3 times 50 ml) and
followed by ethylacetate (3 times 50 ml). The aqueous layer was lyophilized
as an off white hydrochloride salt of 9-[(dimethylamino)methyl]10-
hydroxy(20S)-camptothecin (topotecan hydrochloride) yield 0.236 g (65%).
brand name
Hycamtin
Therapeutic Function
Antineoplastic
Biochem/physiol Actions
Topotecan is a topoisomerase I inhibitor and an apoptosis inducer. It is a potent antineoplastic agent.
Pharmacokinetics
Topotecan, a semi-synthetic derivative of camptothecin (a plant alkaloid obtained from the Camptotheca acuminata tree), is an anti-tumor drug with topoisomerase I-inhibitory activity similar to irinotecan. DNA topoisomerases are enzymes in the cell nucleus that regulate DNA topology (3-dimensional conformation) and facilitate nuclear processes such as DNA replication, recombination, and repair. During these processes, DNA topoisomerase I creates reversible single-stranded breaks in double-stranded DNA, allowing intact single DNA strands to pass through the break and relieve the topologic constraints inherent in supercoiled DNA. The 3'-DNA terminus of the broken DNA strand binds covalently with the topoisomerase enzyme to form a catalytic intermediate called a cleavable complex. After DNA is sufficiently relaxed and the strand passage reaction is complete, DNA topoisomerase reattaches the broken DNA strands to form the unaltered topoisomers that allow transcription to proceed. Topotecan interferes with the growth of cancer cells, which are eventually destroyed. Since the growth of normal cells can be affected by the medicine, other effects may also occur. Unlike irinotecan, topotecan is found predominantly in the inactive carboxylate form at neutral pH and it is not a prodrug.
Clinical Use
Antineoplastic agent:
Treatment of metastatic ovarian, cervical and small cell lung cancer
Metabolism
Topotecan undergoes a reversible pH dependent hydrolysis of its lactone moiety; it is the lactone form that is pharmacologically active.
Metabolism
Topotecan undergoes reversible, pH-dependent hydrolysis of the active lactone moiety to the inactive hydroxyacid (carboxylate) form. A relatively small amount of topotecan is metabolised by hepatic microsomal enzymes to an active metabolite, N-demethyltopotecan; the clinical significance of this metabolite is not known. Excretion is via biliary and renal routes with 20-60
% excreted in the urine as topotecan or the open ring form.
References
[1] creemers gj1, lund b, verweij j. topoisomerase i inhibitors: topotecan and irenotecan. cancer treat rev. 1994 jan;20(1):73-96.
Properties of Topotecan
| Melting point: | −114 °C(lit.) |
| Boiling point: | >100 °C(lit.) |
| Density | 1.2 g/mL at 25 °C(lit.) |
| vapor density | 1.3 (vs air) |
| vapor pressure | 613 psi ( 21.1 °C) |
| Flash point: | 12 °C |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | H2O: soluble |
| form | liquid |
| pka | 8.92±0.40(Predicted) |
| color | yellow |
| CAS DataBase Reference | 123948-87-8(CAS DataBase Reference) |
Safety information for Topotecan
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H340:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Topotecan
Topotecan manufacturer
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Topotecan 99%View Details
Topotecan 99%View Details -
 Topotecan CAS 123948-87-8View Details
Topotecan CAS 123948-87-8View Details
123948-87-8 -
 Hydrogen chloride – 2-propanol solution CASView Details
Hydrogen chloride – 2-propanol solution CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1