123948-87-8
Product Name:
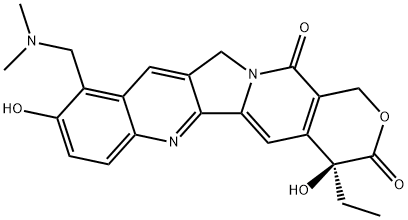
Topotecan
Formula:
C23H23N3O5
Synonyms:
9-[(dimethylamino)methyl]-10-hydroxy-(20S)-camptothecin hydrochloride hydrate;hycamptamine hydrochloride hydrate;Hydrogen chloride solution;NSC-609669 hydrochloride hydrate;SKF-104864A hydrochloride hydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 213-218 °C |
| Solubility | 1 mg/ml |
| LogP | 0.8 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Hazardous decomposition products: Hazardous decomposition products formed under fire conditions. - Carbon oxides, nitrogen oxides (NOx), hydrogen chloride gas. |
| Other Experimental Properties | Light yellow to greenish powder, mp 213-218 °C (decomposes). Soluble in water up to 1 mg/mL. Hygroscopic. Heant and light sensitive /Topotecan hydrochloride/ |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H340:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 421.4 g/mol |
|---|---|
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 421.16377084 g/mol |
| Monoisotopic Mass | 421.16377084 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 867 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Topotecan is a pyranoindolizinoquinoline used as an antineoplastic agent. It is a derivative of camptothecin and works by binding to the topoisomerase I-DNA complex and preventing religation of these 328 single strand breaks. It has a role as an EC 5.99.1.2 (DNA topoisomerase) inhibitor and an antineoplastic agent.