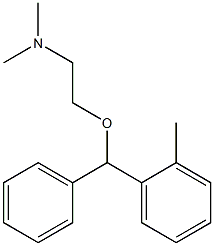
tofenacin
- CAS NO.:15301-93-6
- Empirical Formula: C17H21NO
- Molecular Weight: 255.35
- MDL number: MFCD00865222
- EINECS: 239-338-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
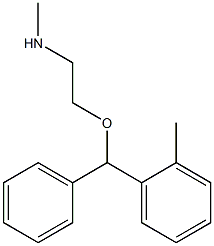
What is tofenacin?
Chemical properties
Off-White Solid
Originator
Elamol,Brocades,UK,1971
The Uses of tofenacin
A metabolite of Orphenadrine. An antidepressant prodrug
The Uses of tofenacin
A metabolite of Orphenadrine (O695300). An antidepressant prodrug
Definition
ChEBI: Tofenacin is a diarylmethane.
Manufacturing Process
A mixture of 39.5 grams of 2-methylbenzhydrol, 200 ml of β-chloroethanol
and 10 ml of concentrated hydrochloric acid is boiled under reflux for 4 hours.
After cooling, the reaction mixture is poured into water and extracted with
petroleum ether (boiling range 40° to 60°C). The layers are separated and the
ethereal solution dried with sodium sulfate. It is then filtered. The filtrate is
concentrated by evaporation of the solvent. The residue is distilled under
reduced pressure to give 51.0 grams (yield 98%) of β-chloroethyl-2-
methylbenzhydryl ether, boiling at 156° to 158°C/2.5 mm.
A mixture of 51 grams of β-chloroethyl-2-methylbenzhydryl ether and 35
grams of methylamine in 140 ml of methanol is heated for 6 hours in a closed
vessel at a temperature of 125° to 135°C. After cooling, the reaction mixture
is poured into water and extracted with petroleum ether (boiling range 40° to
60°C). The ether layer is separated and washed with a 2 N hydrochloric acid
solution. The acidic layer is made alkaline and extracted with ether. The
ethereal solution is separated and dried with sodium sulfate. After filtration,
the solvent is evaporated and the residue distilled under reduced pressure.
There is thus obtained 40 grams (yield 80%) of N-methylaminoethyl-2-
methylbenzhydrylether boiling at 139° to 143°C/0.7 mm.
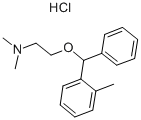
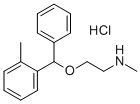
The base is dissolved in anhydrous ether, and an ethereal solution of
hydrochloric acid is added to form the hydrochloride of N-methylaminoethyl-2-
methylbenzhydryl ether. The salt is crystallized from a mixture of ethanol and
ether. Yield is 36 grams (78%); melting point 147° to 148°C.
Therapeutic Function
Psychostimulant
Properties of tofenacin
| Melting point: | <25 °C |
| Boiling point: | bp0.7 139-143° |
| Density | 1.019±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.42±0.10(Predicted) |
| color | Off-White to Pale Yellow |
Safety information for tofenacin
Computed Descriptors for tofenacin
Abamectin manufacturer
Varanous Labs Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![3-Methyl-7-phenyl-6-oxa-3-azabicyclo[6.4.0]dodeca-8,10,12-triene](https://img.chemicalbook.in/CAS/GIF/13669-70-0.gif)
You may like
-
 15301-93-6 N-Desmethyl Orpherdrine 98%View Details
15301-93-6 N-Desmethyl Orpherdrine 98%View Details
15301-93-6 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4