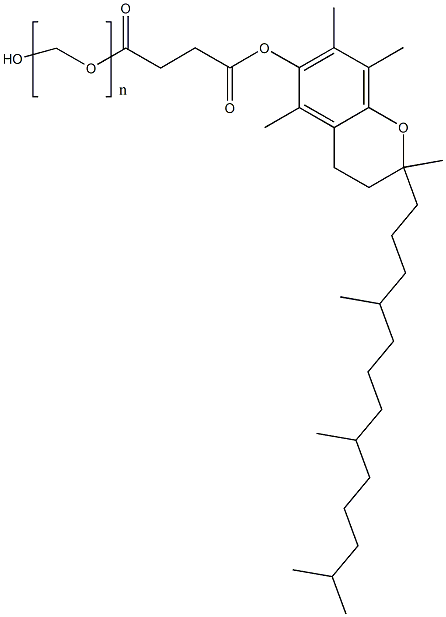
Tocofersolan
Synonym(s):D -α-Tocopherol polyethylene glycol succinate;TPGS;Vitamin E polyethylene glycol succinate;Vitamin E-TPGS
- CAS NO.:9002-96-4
- Empirical Formula: (C2H4O)nC33H54O5
- Molecular Weight: 0
- MDL number: MFCD00146616
- EINECS: 211-836-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Tocofersolan?
Absorption
The bioavailability of vitamin E from tocofersolan is unique from than that of other medicinal products .
Due to its amphipathic property in which it forms its own micelles, tocofersolan is readily taken up into enterocytes, even in the absence of bile salts; fat-soluble d-alpha-tocopherol is then released after hydrolysis. This formulation enhances the absorption of d-alpha-tocopherol compared to the administration of free d-alpha-tocopherol. Additionally, tocophersolan may enhance the absorption of water-insoluble agents and other fat-soluble vitamins .
Tocofersolan is a pro-drug; the active metabolite is the d-alpha-tocopherol. At low concentrations, tocofersolan forms micelles which improve the absorption of non-polar lipids such as other fat-soluble vitamins. Its required micellar concentration is low (0.04 to 0.06 mmol/l) .
A pharmacokinetic study of 12 healthy subjects compared tocofersolan with a water-miscible reference vitamin E after one single oral loading dose of 1200 IU (international units). The relative bioavailability of tocofersolan was found to be (Frel of 1.01 ± 1.74) with AUC0-t of 0.383 ± 0.203μM.h/mg, Cmax of 0.013 ± 0.006, Tmax of 6.0 h (6.0 – 24.0) .
For more information about Vitamin E metabolism, please visit the drug entry Tocopherol.
Toxicity
> 7 g/kg ( Rat )
Common adverse reactions
The most commonly reported adverse reaction during treatment is diarrhea . High doses of Vitamin E may cause diarrhea, abdominal pain, and other gastrointestinal conditions. In the case of an overdose, symptomatic treatment should be provided . High doses of vitamin E have been reported to increase bleeding tendency in patients with Vitamin K deficiency, or patients taking oral anti-vitamins K treatment . Therefore, careful monitoring of the prothrombin time and international normalized ratio (INR) are advised. A possible adjustment of the dose of oral anticoagulant during and after treatment with Vedrop may be necessary .
Renal impairment
Data regarding patients with renal impairment are limited, this drug should be administered with caution and those with renal impairment or dehydration should be closely followed .
Hepatic impairment
This drug should be administered with caution in patients with liver impairment and under close monitoring of the liver function tests in such patients .
Hypersensitivity
Vedrop, the commercial form, contains sodium methyl parahydroxybenzoate (E219) and sodium ethyl parahydroxybenzoate (E215) which may cause allergic reactions, which are sometimes delayed .
Pregnancy
There is no current data on taking tocofersolan during pregnancy. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/ fetal development, parturition or postnatal development. Caution should be taken when prescribing this medication to pregnant women .
Breast-feeding
It is unknown whether tocofersolan is released into human breast milk. The excretion of tocofersolan in milk has not been studied in animals .
Fertility
No data is available .
Chemical properties
Vitamin E polyethylene glycol succinate is a synthetic product. It is available as a white to light-brown, waxy solid and is practically tasteless. Chemically, it is a mixture composed principally of monoesterified polyethylene glycol 1000, the diesterified polyethylene glycol 1000, free polyethylene glycol 1000, and free tocopherol.
The Uses of Tocofersolan
Tocofersolan is a polyethylene glycol derivative of α-Tocopherol (T526125). Tocofersolan is a synthetic water-soluble vitamin E unlike its natural counterpart which are fat soluble. Tocofersolan is used in pediatrics in the treatment vitamin E-deficient cholestatic children. Tocofersolan has potential application as a absorption enhancer in drug delivery systems.
The Uses of Tocofersolan
tocophersolan is an anti-oxidant, this is a water-soluble form of vitamin e.
The Uses of Tocofersolan
Tocofersolan is a polyethylene glycol derivative of α-Tocopherol (T526125). Tocofersolan is a synthetic water-soluble vitamin E unlike its natural counterpart which are fat soluble. Tocofersolan is used in pediatrics in the treatment vitamin E-deficient cholestatic children. Tocofersolan has potential application as a absorption enhancer in drug delivery systems. Typical batch, n = 21
Background
D-Alpha-tocopheryl polyethylene glycol 1000 succinate (Tocofersolan, Vedrop), has been developed in Europe as an orally bioavailable source of vitamin E in children suffering from cholestasis . Cholestasis is the reduction or stoppage of bile flow, either to impaired secretion by hepatocytes (liver cells) or obstruction , .
Tocofersolan is a polyethylene glycol derivative of α-tocopherol and synthetic water-soluble version of Tocopherol. Tocofersolan is an oral treatment of vitamin E deficiency due to digestive malabsorption in pediatric patients with congenital chronic cholestasis or hereditary chronic cholestasis. It was approved by the European Medicines Agency (EMA) in June 2009 under the market name Vedrop. Moreover, the agent is capable of demonstrating antioxidant effects that make it a popular component to include in cosmetics and pharmaceuticals as well.
In addition to the above, tocofersolan has been studied as a promising application as an absorption enhancer in drug delivery .
Indications
Tocofersolan is indicated in vitamin E deficiency caused by digestive malabsorption in pediatric patients with congenital chronic cholestasis or hereditary chronic cholestasis from birth (full term newborns) up to 18 years of age .
Production Methods
Vitamin E polyethylene glycol succinate is prepared by esterification of the acid group of crystalline D-a-tocopheryl acid succinate by polyethylene glycol 1000.
Pharmaceutical Applications
Vitamin E polyethylene glycol succinate is an esterified vitamin E
(tocopherol) derivative primarily used as a solubilizer or emulsifying
agent because of its surfactant properties. Structurally, it is
amphipathic and hydrophilic, unlike the tocopherols, and therefore
it is a water-soluble derivative that can be used in pharmaceutical
formulations such as capsules, tablets, hot-melt extrusion,
microemulsions, topical products, and parenterals. One of
the most important applications is its use as a vehicle for lipid-based
drug delivery formulations. It can also be used as a source of
vitamin E.
Vitamin E polyethylene glycol succinate has been characterized
with respect to its mechanism of action and studied as a Pglycoprotein
inhibitor.
Biochem/physiol Actions
D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS) also known as vitamin E TPGS, is a therapeutic agent for vitamin E deficiency in children, administered during chronic childhood cholestasis. TPGS exerts anti-tumor activity in MCF-7 and breast cancer cells by downregulating anti-apoptotic proteins. TPGS finds its application as an emulsifier in the preparation of poly(lactic-co-glycolic acid) (PLGA) particles for DNA labelling, produces particles of uniform size, high encapsulation efficiency, increases hydrophilicity and prevents aggregation of particles.
Pharmacokinetics
Because of its increased solubility , , , this medication readily penetrates cells, unlike other types of vitamin E , which are strictly fat-soluble . Specific to cholestasis, tocofersolan normalizes vitamin E levels relieving the symptoms of deficiency because of its facilitation of vitamin E absorption , .
Safety
Vitamin E polyethylene glycol succinate has been used at levels of 280 mg/capsule in the product Agenerase (amprenavir), which was dosed at 8 capsules (2240mg vitamin E TPGS) per day. An additional assessment of the safety of vitamin E polyethylene glycol succinate has been published, which includes a report showing no-observed-adverse-effect-level (NOAEL) in rats of 1000 mg/kg/day.
Metabolism
The hydrolysis of tocofersolan occurs in the gut lumen. Tocofersolan is absorbed by cells, and the alpha-tocopherol moiety appears in chylomicrons in the lymph system in a manner that is identical to vitamin E absorbed from dietary sources. Cellular uptake does not require receptors, binding proteins or metabolic processes and does not occur by pinocytosis. Absorption of deuterated tocofersolan demonstrated a normal pattern in lipoproteins: alpha-tocopherol peaked first in chylomicrons, then peaked in very low- density lipoproteins (VLDL) and finally in low-density lipoproteins (LDL) and high-density lipoproteins (HDL).
storage
Vitamin E polyethylene glycol succinate is stable at ambient room temperature for up to 4 years. It reacts with alkalis and acids. Aqueous solutions of vitamin E polyethylene glycol succinate are stable over a pH range of 4.5–7.5 and can be further stabilized with propylene glycol.
Incompatibilities
Vitamin E polyethylene glycol succinate is incompatible with strong acids and strong alkalis.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (ophthalmic solution or drops; oral capsules, solution, tablet; topical solution or drops). Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Tocofersolan
| Melting point: | 34-38°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble1g/10 mL, clear to faintly turbid, colorless to faintly yellow |
| form | Solid |
| color | White to Pale Yellow Waxy |
| InChI | InChI=1S/C34H56O6/c1-23(2)12-9-13-24(3)14-10-15-25(4)16-11-20-34(8)21-19-29-28(7)32(26(5)27(6)33(29)40-34)39-31(37)18-17-30(36)38-22-35/h23-25,35H,9-22H2,1-8H3 |
Safety information for Tocofersolan
Computed Descriptors for Tocofersolan
| InChIKey | WFWQYXBTWIHIIW-UHFFFAOYSA-N |
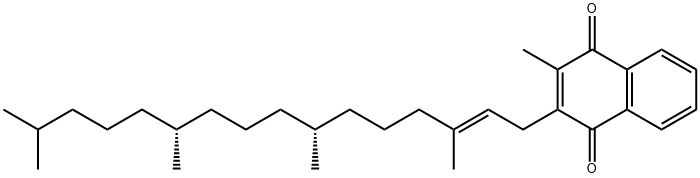
| SMILES | C(=O)(OC1C(C)=C2CCC(C)(CCCC(CCCC(CCCC(C)C)C)C)OC2=C(C)C=1C)CCC(=O)OCO |
Tocofersolan manufacturer
GLR Innovations
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Tocofersolan pharm solubilizerView Details
Tocofersolan pharm solubilizerView Details
9002-96-4 -
 Tocofersolan Vitamin E PolyethyleneGlycol Succinate pharmaceutic adjuvantView Details
Tocofersolan Vitamin E PolyethyleneGlycol Succinate pharmaceutic adjuvantView Details
9002-96-4 -
 9002-96-4 / 162849-98-1 Tocophersolan 98%View Details
9002-96-4 / 162849-98-1 Tocophersolan 98%View Details
9002-96-4 / 162849-98-1 -
 Tocofersolan CAS 9002-96-4View Details
Tocofersolan CAS 9002-96-4View Details
9002-96-4 -
 α-Tocopherol Polyethylene Glycol Succinate CAS 9002-96-4View Details
α-Tocopherol Polyethylene Glycol Succinate CAS 9002-96-4View Details
9002-96-4 -
 D-α-Tocopherol polyethylene glycol 1000 succinate CAS 9002-96-4View Details
D-α-Tocopherol polyethylene glycol 1000 succinate CAS 9002-96-4View Details
9002-96-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1