VitaMin D4
Synonym(s):Cholecalciferol;Calciol;Vitamin D4;(+)-Vitamin D3;(3β,5Z,7E)-9,10-Secoergosta-5,7,10(19)-trien-3-ol
- CAS NO.:511-28-4
- Empirical Formula: C28H46O
- Molecular Weight: 398.66
- MDL number: MFCD18781880
- EINECS: 208-127-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 16:13:33
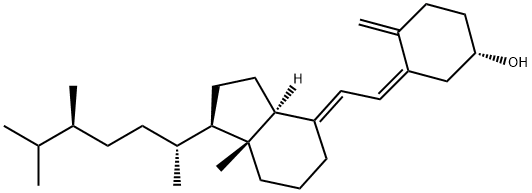
What is VitaMin D4?
Description
Derived from cholesterol, vitamin D is biosynthesized from its prohormone cholecalciferol (D3), the product of solar ultraviolet irradiation of 7-dehydrocholesterol in the skin. In 1966, it was first recognized that vitamin D must undergo activation via two oxidative metabolic steps. The first oxidation to 25-hydroxycholecalciferol (25(OH)D3: calcifediol; Calderol) occurs in the endoplasmic reticulum of the liver and is catalyzed by vitamin D 25-hydroxylase. This activation step is not regulated by plasma calcium concentrations. The major circulating form (10–80 μg/mL) is 25(OH)D3, which also is the primary storage form of vitamin D.
Chemical properties
Pale Yellow Oil
The Uses of VitaMin D4
Vitamin D4 is the active analogue of Vitamin D.
Definition
ChEBI: Vitamin D4 is it is present in mushrooms. It has a role as a fungal metabolite. It is a vitamin D and a seco-ergostane.
Biological Functions
Sterol-specific cytoplasmic receptor proteins (vitamin D receptor) mediate the biological action of vitamin D. The active hormone is transported from the cytoplasm to the nucleus via the vitamin D receptor, and as a result of the interaction of the hormone with target genes, a variety of proteins are produced that stimulate the transport of calcium in each of the target tissues Active vitamin D works in concert with PTH to enhance active intestinal absorption of calcium, to stimulate bone resorption, and to prohibit renal excretion of calcium. If serum calcium or 1,25-calcitriol concentrations are elevated, then vitamin D 24-hydroxylase (in renal mitochondria) is activated to oxidize 25(OH)D3 to inactive 24,25-dihydroxy-cholecalciferol and to further oxidize active vitamin D to the inactive 1,24,25-trihydroxylated derivative. Both the 1,24,25-trihydroxylated and the 24,25-dihydroxylated products have been found to suppress PT H secretion as well. The biosynthesis of vitamin D is tightly regulated based on the serum concentrations of calcium, phosphate, PTH, and active vitamin D.
Properties of VitaMin D4
| Melting point: | 83-86 °C(lit.) |
| alpha | D18 +89.3° (c = 0.47 in acetone) |
| Boiling point: | 504.9±29.0 °C(Predicted) |
| Density | 0.96 |
| storage temp. | 2-8°C |
| solubility | Soluble in Chloroform |
| form | Powder |
| pka | 14.74±0.20(Predicted) |
| color | Off-white to light yellow |
| Stability: | Hygroscopic, Temperature Sensitive |
| CAS DataBase Reference | 511-28-4(CAS DataBase Reference) |
Safety information for VitaMin D4
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H330:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for VitaMin D4
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
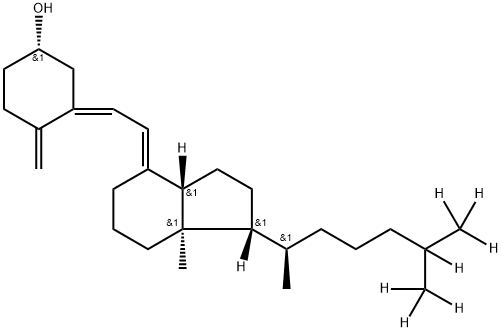
You may like
-
 Vitamin D, 25Kg DrumView Details
Vitamin D, 25Kg DrumView Details
67-97-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
