Tibolone
Synonym(s):(7α,17α)-17-Hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one;17-Hydroxy-7α-methyl-19-nor-17α-pregn-5(10)-en-20-yn-3-one;5(10)-Estren-7α-methyl-17α- ethynyl-17β- ol-3-one
- CAS NO.:5630-53-5
- Empirical Formula: C21H28O2
- Molecular Weight: 312.45
- MDL number: MFCD00864178
- EINECS: 227-069-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-18 17:18:22
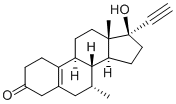
What is Tibolone?
Absorption
Tibolone is extensively and rapidly absorbed after oral administration . The parent drug undergoes extensive metabolism, with. Greater than 80% of a radioactive dose excreted from the body as metabolites, which suggests very low plasma concentrations of tibolone. Plasma concentrations of the metabolites appear within 30 minutes and peak within 1–1.5 hours.2,7 The plasma concentrations of the hydroxymetabolites are higher than those of the ?4-isomer. Food does not appear to have an effect on the absorption of this drug .
Toxicity
>2000 mg/kg
The Million Women Study (MWS), which had a prospective observational design, studied the use of hormone replacement therapy. The results indicated that the increase in the incidence of breast cancer with estrogen and progestogen (compared to estrogen alone) was greater than the reduction in occurrence of endometrial cancer associated with adding progestogen to estrogen therapy. The MWS also reported a marked increase in the incidence of breast cancer with tibolone and with implanted and transdermal estrogen-only preparations .
Tibolone treatment in rodent studies showed an increased association with the development of a range of tumors in long-term oral carcinogenicity studies. These tumors included pituitary adenomas, mammary carcinomas and fibroadenomas, hepatic adenomas, uterine carcinoma, stromal polyps and stromal sarcoma, and carcinomas of the urinary bladder and testes. Tibolone failed to show any evidence of genotoxicity in studies for gene mutations, chromosomal damage as well as DNA damage .
Other adverse effects these include dizziness, headache, nausea, abdominal pain, rashes, pruritus, weight gain, edema, and migraine .
Description
Tibolone is a synthetic steroid with weak progestational and estrogenic properties, reportedly useful in controlling symptoms resulting from natural or surgical menopause. It has thus far shown no significant antithrombotic effect in post-menopausal patients.
Chemical properties
White Solid
Originator
Akzo (Organon) (The Netherklands)
The Uses of Tibolone
A synthetic steroid with weak estrogenic, androgenic and progestogenic activity. A pharamceutical used in the treatment of menopausal syndrome
Indications
For the relief of post-menopausal symptoms and for the prevention of osteoporosis .
Background
Tibolone is a synthetic steroid hormone drug, which is mainly non-selective in its binding profile, acting as an agonist primarily at estrogen receptors (ER), with a preference for ER alpha .
Tibolone (Livial, Org OD 14), produced by Organon (West Orange, NJ), is a synthetic steroid that possesses estrogenic, androgenic and progestogenic properties. It has been used in Europe for almost 2 decades, primarily for the prevention of postmenopausal osteoporosis and the treatment of post-menopausal symptoms . Tibolone is approved in 90 countries to manage menopausal symptoms and in 45 countries to prevent the development of osteoporosis .
In June 2006, Organon Pharmaceuticals announced the receipt of a Not Approvable Letter from the U.S. Food and Drug Administration (FDA), advising the company that the New Drug Application (NDA) for tibolone had not been approved .
Interestingly, the use of tibolone in the treatment cardiovascular disease has been studied with inconclusive results . Tibolone has been to have anti-resorptive effects on bone .
Definition
ChEBI: Estran-3-one with a double bond between positions 5 and 10, and bearing both an ethynyl group and a hydroxy group at position 17 (R-configuration). A synthetic steroid hormone drug which acts as an agonist at all five type I steroid hormon receptors, it is used in the prevention of postmenopausal osteoporosis and for treatment of endometriosis.
brand name
Livial
Pharmacokinetics
Tibolone prevents bone loss and treating post-menopausal symptoms without stimulating the endometrial tissues, which may lead to malignancy. Typical, drugs that treat post-menopausal symptoms such as estrogen, have a proliferative effect on the endometrium, increasing the risk of endometrial carcinoma . The effects on the bone, brain and vaginal tissues can be explained by the estrogenic activity of tibolone. It is important to note that activity is not expressed in the endometrium. Tibolone behaves differently from estrogen plus progesterone combinations on the breast. Therefore, tibolone can be characterized as a selective estrogen activity regulator .
Tibolone has been demonstrated to be an effective agent in treating symptoms associated with menopause. A 16 week trial in 1189 women examined the effect of tibolone 2.5 mg once daily on climacteric symptoms. Women treated with tibolone showed improvement from baseline in typical menopausal symptoms including hot flashes, sweating, insomnia, and anxiety .
Pharmacokinetics
Raloxifene is rapidly absorbed following oral administration, with an estimated 60% absorption, but it has a very low bioavailability (2%), associated with extensive phase II metabolism. The metabolites are excreted via the bile, with potential enterohepatic recycling that could account for the interaction with cholestyramine. Supportive of the enterohepatic recycling is the half-life of 28 hours. Metabolism of raloxifene occurs to a great extent in the intestine and consists of glucuronide conjugation catalyzed by uridine diphosphate glucuronosyltransferase (UGT).
Clinical Use
Tibolone is a synthetic steroid that has been shown to increase bone mineral density similar to alendronate. The U.S. FDA approval is pending; overseas, this agent is used for the treatment of menopausal symptoms as well as the prevention of osteoporosis. It is considered to be a viable alternative to conjugated equine estrogen plus micronized progesterone.
Side Effects
The most common reason for patient withdrawal from clinical trials was vaginal bleeding (also a common side effect when estrogen therapy is used).
Metabolism
Tibolone is metabolized mainly in the liver .
The cytochrome P450 isoenzyme system is involved in minor hydroxylation of tibolone .
Tibolone is rapidly converted into three major metabolites: 3 alpha- and 3 beta-hydroxy-tibolone, which have oestrogenic effects, and the Delta(4)-isomer, which has both progestogenic and androgenic effects. The 3-hydroxy metabolites are present in the circulation, predominantly in their inactive sulfated form .
Properties of Tibolone
| Melting point: | 169 °C |
| Boiling point: | 447.4±45.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| refractive index | 105 ° (C=0.54, CH2Cl2/Et2O) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble15mg/mL, clear |
| pka | 13.10±0.60(Predicted) |
| form | neat |
| color | white to beige |
| Merck | 9427 |
| InChI | InChI=1/C21H28O2/c1-4-21(23)10-8-18-19-13(2)11-14-12-15(22)5-6-16(14)17(19)7-9-20(18,21)3/h1,13,17-19,23H,5-12H2,2-3H3/t13-,17-,18+,19-,20+,21+/s3 |
| CAS DataBase Reference | 5630-53-5(CAS DataBase Reference) |
Safety information for Tibolone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Tibolone
| InChIKey | WZDGZWOAQTVYBX-PGFZCDQENA-N |
| SMILES | [C@@]12([H])[C@H](C)CC3CC(=O)CCC=3[C@@]1([H])CC[C@]1(C)[C@](CC[C@@]21[H])(O)C#C |&1:0,2,12,16,18,21,r| |
Tibolone manufacturer
Swati Spentose Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 Tibolone 99%View Details
Tibolone 99%View Details -
 5630-53-5 98%View Details
5630-53-5 98%View Details
5630-53-5 -
 Tibolone 99% (HPLC) CAS 5630-53-5View Details
Tibolone 99% (HPLC) CAS 5630-53-5View Details
5630-53-5 -
 5630-53-5 98%View Details
5630-53-5 98%View Details
5630-53-5 -
 Tibolone 5630-53-5 99%View Details
Tibolone 5630-53-5 99%View Details
5630-53-5 -
 5630-53-5 Tibolone 99%View Details
5630-53-5 Tibolone 99%View Details
5630-53-5 -
 Tibolone 95.00% CAS 5630-53-5View Details
Tibolone 95.00% CAS 5630-53-5View Details
5630-53-5 -
 Tibolone CAS 5630-53-5View Details
Tibolone CAS 5630-53-5View Details
5630-53-5