Empenthrin
- CAS NO.:54406-48-3
- Empirical Formula: C18H26O2
- Molecular Weight: 274.4
- MDL number: MFCD01745518
- EINECS: 259-154-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43
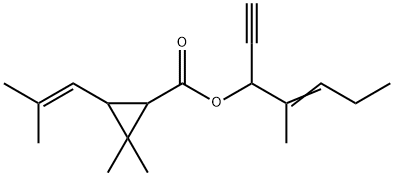
What is Empenthrin?
Description
Empenthrin, also known as vaporthrin, (2E)-1-ethynyl-2-methyl-2-penten-1-yl 2,2- dimethyl-3-(2-methyl-1-propen-1-yl) cyclopropanecarboxylate, is a derivative of 1-ethynyl- 2-methyl penten-2-ol. The vapor pressure of empenthrin at room temperature is suitable and therefore it has a natural volatility. At 30 °C, the vapor pressures of empenthrin and allethrin are 1.62?×?10?3 and 1.20?×?10?4 mmHg, respectively. Thus, the vapor pressure of empenthrin is about 10 times more than that of allethrin. Moreover, empenthrin is odorless. It has strong killing activity and repellent effect on pests and is very suitable for textile mothproofing.
Chemical properties
Empenthrin is a light yellow and oily liquid with a boiling point of 346°C and a flash point of 144°C. Its density is 0.999 g.cm–3 at 20°C. The refractive index of empenthrin is 1.5270 at 20°C. It has low acute mammalian toxicity (its oral LD50 is >5,000 mg.kg–1 in male rats, >3,500 mg.kg–1 in female rats and >3,500 mg.kg–1 in mice). It is, however, very toxic to fish and other aquatic organisms.
The Uses of Empenthrin
Empenthrin is used as a domestic insecticide, especially against moths and other insects which attack fabrics.
The Uses of Empenthrin
Empenthrin is an insecticidal pyrethroid compound.
What are the applications of Application
Empenthrin is an insecticidal pyrethroid compound
Definition
ChEBI: Empenthrin is a monoterpenoid.
Preparation
About 3.72 g (0.03 mol) of 2-methyl-1-ethynyl-2-pentenol and 3.16 g (0.04 mol) of pyridine were added to toluene to form a solution. A toluene solution containing 5.6 g (0.03 mol) of Chrysanthemoyl chloride was added in a dropwise manner. The reaction was carried out at a temperature of 40–50°C for 6h. After reaction, the reaction solution was washed with water until neutral, dried and then subjected to distillation under reduced pressure. About 5.7 g of empenthrin was collected from a fraction of 120–126°C (0.03 mmHg). The yield of empenthrin was 69%.
Metabolic pathway
Empenthrin is a low molecular weight ester of (1R)-chrysanthemic acid (2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylic acid). It has a relatively high vapour pressure and, unlike most pyrethroids, it is effective against flying insects. The molecule has three chiral centres and geometric isomerism in the alcohol group but, with the exception of the 1R acid, the full isomer mixture is used. As the insecticide has been developed for domestic use, it is unlikely that there will be a requirement for metabolism studies in plants or for extensive environmental fate studies. Its metabolism in rats has been reported, with particular attention paid to the fate of the novel alcohol moiety. As with other pyrethroids, ester cleavage and oxidation and conjugation of the alcohol metabolite predominated. The fate of the chrysanthemic acids can be assessed by reference to phenothrin.
Degradation
Empenthrin is a stable molecule but it would be expected to be labile at pH values above 10.
Properties of Empenthrin
| Melting point: | 25°C |
| Boiling point: | 377.38°C (rough estimate) |
| Density | 0.9965 (rough estimate) |
| vapor pressure | 1.4×10-2 Pa (23.6 °C) |
| refractive index | 1.5510 (estimate) |
| storage temp. | 2-8°C |
| Water Solubility | 0.11 mg l-1 (25 °C) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 54406-48-3(CAS DataBase Reference) |
Safety information for Empenthrin
Computed Descriptors for Empenthrin
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
