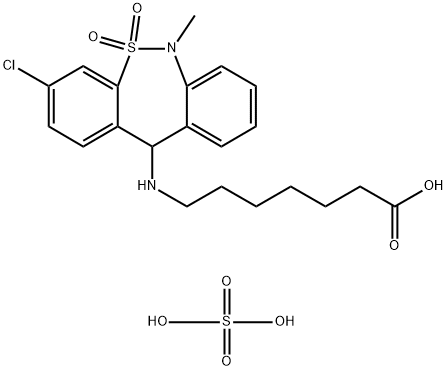
Tianeptine
- CAS NO.:66981-73-5
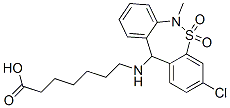
- Empirical Formula: C21H25ClN2O4S
- Molecular Weight: 436.95
- MDL number: MFCD00865376
- EINECS: 614-004-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Tianeptine?
Description
Tianeptine, a tricyclic compound, is a selective facilitator of 5-HT (serotonin; sc-201146) uptake in vitro and in vivo. Additionally, Tianeptine is reported to act as a selective inhibitor of dopamine uptake and is similar to amineptine. Tianeptine does not have a noted effect on monoamine uptake.
Chemical properties
White Solid
Originator
Stablon,Servier,France,1983
The Uses of Tianeptine
Tianeptine is used in the treatment of reactive and neurotic depression, as well as in the treatment of anxious states.
The Uses of Tianeptine
Tricyclic compound with psychostimulant, anti-ulcer and anti-emetic properties. Antidepressant
What are the applications of Application
Tianeptine is a selective facilitator of serotonin uptake
Definition
ChEBI: Tianeptine is a racemate comprising of equimolar amounts of (R)- and (S)-tianeptine. It is an atypical antidepressant used in Europe to treat patients who respond poorly to selective serotonin reuptake inhibitors (SSRIs). It has a role as a mu-opioid receptor agonist, a second generation antipsychotic and an anxiolytic drug. It contains a (R)-tianeptine and a (S)-tianeptine.
Manufacturing Process
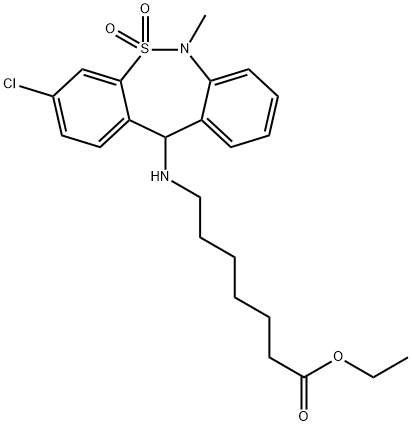
A solution of 27.6 g (0.16 mol) of freshly distilled ethyl 7-aminoheptanoate in
40 ml of nitromethane was added all at once and with mechanical stirring to a
suspension of 26.2 g (0.08 mol) of 5,8-dichloro-10-dioxo-11-
methyldibenzo[c,f]thiazepine(1,2) in 120 ml of nitromethane. The whole was
heated to 55°C for 30 minutes, the solvent was then evaporated in vacuo and
the residue was taken up in water. The crude ester was extracted with ether.
After evaporation of the ether 36 g of crude ester were obtained, and 30 g
(0.065 mol) there of were treated under reflux with a solution of 2.8 g (0.07
mol) of sodium hydroxide in 35 ml of ethanol and 25 ml of water. After one
hour's refluxing, the alcohol was evaporated in vacuo. The residue was taken
up in 150 ml of water.
The mixture was twice extracted with 75 ml of chloroform and the aqueous
phase was evaporated in vacuo. The sodium salt was then dissolved in 150 ml
of chloroform, the solution was dried over sodium sulfate and the product
precipitated with anhydrous ether.
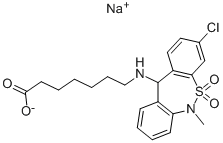
The salt was filtered off, washed with ether and dried at 50°C. 13 g of sodium
7-[8-chloro-10-dioxo-11-methyldibenzo[c,f]thiazepin-(1,2)-aminoheptanoate,melting with decomposition at about 180°C, were obtained.
Therapeutic Function
Antidepressant
Biological Activity
Tianeptine was identified as an efficacious MOR full agonist, binding to the human MOR with a Ki of 383 ± 183 nM. Tianeptine was also found to act as a full agonist at the DOR, although it had much lower potency and was inactive at the KOR. The MOR was required for the acute and chronic antidepressant-like effects of tianeptine in mice. Although tianeptine also produced behavioural effects, including analgesia and reward, its effects were distinct from morphine in that it did not produce tolerance or withdrawal.
Properties of Tianeptine
| Melting point: | 129-131°C |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Freely soluble in water, in methanol and in methylene chloride. |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 66981-73-5(CAS DataBase Reference) |
Safety information for Tianeptine
Computed Descriptors for Tianeptine
| InChIKey | JICJBGPOMZQUBB-UHFFFAOYSA-N |
| SMILES | C(O)(=O)CCCCCCNC1C2=CC=C(Cl)C=C2S(=O)(=O)N(C)C2=CC=CC=C21 |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4