2-Thiophenethiol
Synonym(s):2-Mercaptothiophene;Thienylmercaptan
- CAS NO.:7774-74-5
- Empirical Formula: C4H4S2
- Molecular Weight: 116.2
- MDL number: MFCD00051666
- EINECS: 231-881-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-09 16:05:57
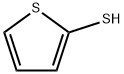
What is 2-Thiophenethiol?
Chemical properties
Clear yellowish to orange liquid
Chemical properties
2-Thienyl mercaptan has a very unpleasant, burnt caramellic and sulfuraceous odor with a similar flavor.
The Uses of 2-Thiophenethiol
2-Thiophenethiol is used in biological studies to evaluate the changes in key odorants of hazelnut induced by roasting.
What are the applications of Application
2-Mercaptothiophene is a thiophene derivative used in biochemical research
Preparation
By heating sodium sulfosuccinate with phosphorous trichloride; also by reduction of thiophene-2-sulfonyl chloride.
Definition
ChEBI: Thiophene-2-thiol is a heteroarene.
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 6316, 1953 DOI: 10.1021/ja01120a518
Synthesis
A three-necked flask fitted with a mechanical stirrer and a 600-ml dropping funnel filled with dry nitrogen is charged with 500 ml of tetrahydrofuran and 56 g (53 ml, 0.67 mole) of thiophene. This mixture is stirred under nitrogen and cooled to ?40° with an acetone–dried ice bath while 490 ml (0.662 mole) of 1.35 M n-butyllithium in pentane is added over 5 minutes via the dropping funnel. The mixture's temperature is held between ?30° and ?20° for 1 hour, then lowered to ?70° by adding dry ice to the bath. Powdered sulfur crystals (20.4 g, 0.638 g) are added to the stirred mixture in one aliquot. After 30 minutes, the temperature is allowed to rise to ?10°, and then the yellow solution is carefully poured into 1 l of rapidly stirred ice water. These aqueous extracts are combined with the aqueous layer, and the whole is chilled and carefully acidified with 4 N sulfuric acid. This aqueous phase is immediately extracted with three 200-ml portions of diethyl ether. The combined ether extracts are washed twice with 100 ml portions of water to remove acid and remaining tetrahydrofuran and dried over anhydrous sodium sulfate. After the removal of ether, the residual golden-brown oil is purified by distillation at reduced pressure. The portion boiling at 53–56° (5 mm) is collected, yielding 49.5–53.5 g (65–70%) of 2-thiophenethiol as a yellow oil.
Properties of 2-Thiophenethiol
| Boiling point: | 129 °C(lit.) |
| Density | 1.252 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 3062 | 2-THIENYL MERCAPTAN |
| Flash point: | 150 °F |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | soluble in Chloroform, DMSO |
| form | clear liquid |
| pka | 6.38±0.43(Predicted) |
| color | Light yellow to Brown |
| Odor | at 0.10 % in dipropylene glycol. burnt caramel roasted coffee |
| Sensitive | Air Sensitive |
| JECFA Number | 1052 |
| BRN | 104650 |
| CAS DataBase Reference | 7774-74-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Thiophenethiol(7774-74-5) |
Safety information for 2-Thiophenethiol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Thiophenethiol
| InChIKey | SWEDAZLCYJDAGW-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2-Thiophenethiol CAS 7774-74-5View Details
2-Thiophenethiol CAS 7774-74-5View Details
7774-74-5 -
 2-Thiophenethiol, ≥98% CAS 7774-74-5View Details
2-Thiophenethiol, ≥98% CAS 7774-74-5View Details
7774-74-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1