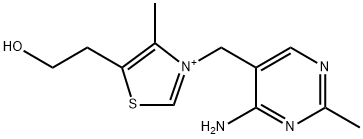
THIAMINE HYDROCHLORIDE
- CAS NO.:70-16-6
- Empirical Formula: C12H17N4OS+
- Molecular Weight: 265.35
- MDL number: MFCD00012780
- EINECS: 200-641-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-10 17:18:44
What is THIAMINE HYDROCHLORIDE?
Absorption
Absorbed mainly from duodenum, by both active and passive processes
Toxicity
Thiamine toxicity is uncommon; as excesses are readily excreted, although long-term supplementation of amounts larger than 3 gram have been known to cause toxicity. Oral mouse LD50 = 8224 mg/kg, oral rat LD50 = 3710 mg/kg.
Physical properties
Free thiamin is unstable because of its quaternary nitrogen; in water, it is cleaved to the thiol form. For this reason, the hydrochloride and mononitrate forms are used in commerce. Thiamin hydrochloride (actually, thiamin chloride hydrochloride) is a colorless crystal that is very soluble in water (1 g/ml, thus making it a very suitable form for parenteral administration), soluble in methanol and glycerol, but practi cally insoluble in acetone, ether, chloroform, and benzene. The mononitrate form is more stable than the hydrochloride form, but it is less soluble in water. It is used in food/feed supplementation and in dry pharmaceutical preparations. Free thiamin is easily oxidized to thiamin disulfide and other derivatives, including thiochrome, a yellow biologically inactive product with strong blue fluorescence that can be used for the quantitative determination of thiamin. Its metabolically active form is thia min diphosphate, also called thiamin pyrophosphate.
Background
Thiamine or thiamin, also known as vitamin B1, is a colorless compound with the chemical formula C12H17N4OS. It is soluble in water and insoluble in alcohol. Thiamine decomposes if heated. Thiamine was first discovered by Umetaro Suzuki in Japan when researching how rice bran cured patients of Beriberi. Thiamine plays a key role in intracellular glucose metabolism and it is thought that thiamine inhibits the effect of glucose and insulin on arterial smooth muscle cell proliferation. Thiamine plays an important role in helping the body convert carbohydrates and fat into energy. It is essential for normal growth and development and helps to maintain proper functioning of the heart and the nervous and digestive systems. Thiamine cannot be stored in the body; however, once absorbed, the vitamin is concentrated in muscle tissue.
Indications
For the treatment of thiamine and niacin deficiency states, Korsakov's alcoholic psychosis, Wernicke-Korsakov syndrome, delirium, and peripheral neuritis.
Definition
ChEBI: Thiamine(1+) is a primary alcohol that is 1,3-thiazol-3-ium substituted by (4-amino-2-methylpyrimidin-5-yl)methyl, methyl and 2-hydroxyethyl groups at positions 3, 4 and 5, respectively. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a primary alcohol and a vitamin B1. It is a conjugate base of a thiamine(2+).
Pharmacokinetics
Thiamine is a vitamin with antioxidant, erythropoietic, cognition-and mood-modulatory, antiatherosclerotic, putative ergogenic, and detoxification activities. Thiamine has been found to protect against lead-induced lipid peroxidation in rat liver and kidney. Thiamine deficiency results in selective neuronal death in animal models. The neuronal death is associated with increased free radical production, suggesting that oxidative stress may play an important early role in brain damage associated with thiamine deficiency. Thiamine plays a key role in intracellular glucose metabolism and it is thought that thiamine inhibits the effect of glucose and insulin on arterial smooth muscle cell proliferation. Inhibition of endothelial cell proliferation may also promote atherosclerosis. Endothelial cells in culture have been found to have a decreased proliferative rate and delayed migration in response to hyperglycemic conditions. Thiamine has been shown to inhibit this effect of glucose on endothelial cells.
Metabolism
Hepatic
Properties of THIAMINE HYDROCHLORIDE
| Melting point: | 250 °C (dec.)(lit.) |
| storage temp. | 2-8°C |
| solubility | H2O: 0.1 g/mL at 20 °C, clear, colorless |
Safety information for THIAMINE HYDROCHLORIDE
Computed Descriptors for THIAMINE HYDROCHLORIDE
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran




You may like
-
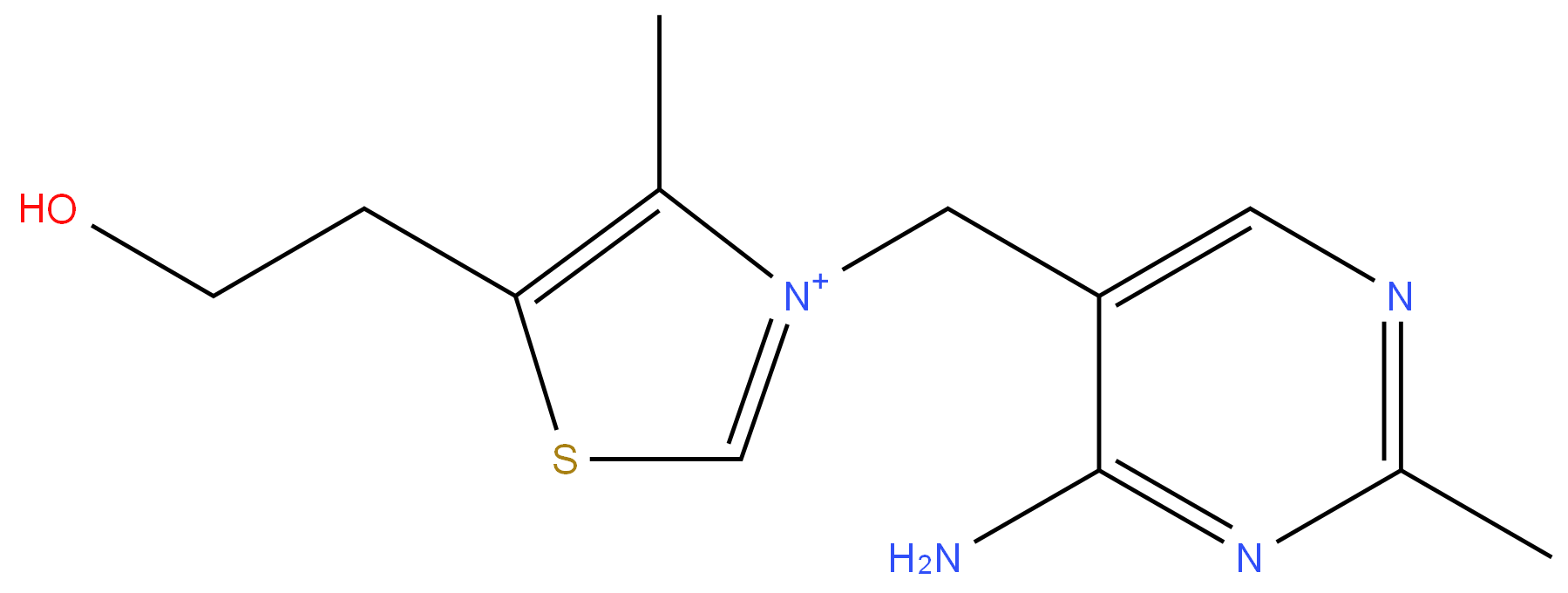 Vitamin B1 HCL 99%View Details
Vitamin B1 HCL 99%View Details -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
