THALLIUM(I) SULFATE
- CAS NO.:7446-18-6
- Empirical Formula: O4STl2
- Molecular Weight: 504.83
- MDL number: MFCD00011278
- EINECS: 231-201-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
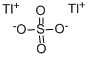
What is THALLIUM(I) SULFATE?
Chemical properties
white fine crystalline powder or needles
The Uses of THALLIUM(I) SULFATE
As rat poison, as ant bait and as a reagent in analytical chemistry.
The Uses of THALLIUM(I) SULFATE
Thallium(I) sulfate is used as a precursor to thallium(I) sulfide, which exhibits high electrical conductivity when exposed to infrared light. It is further used in photocells. It is also mixed with selenium and arsenic to produce low melting glasses.
Production Methods
Commercial sources are flue dusts, either from pyrite (FeS2) burners or from lead and zinc smelters and refiners, as a byproduct of cadmium production at the rate of a few thousand pounds per year. In the flue dusts thallium occurs largely as a sulfate, which is extracted with hot water or dilute sulfuric acid. The purification of thallium is accomplished by taking advantage of the difference in solubility of certain thallium compounds and the impurities. Traces of zinc, copper, cadmium, lead, and indium are removed by dissolving the thallium in and precipitating the impurities with hydrogen sulfide.
Definition
ChEBI: Thallium sulfate is a metal sulfate in which the counterion is thallium and the ratio of thallium to sulfate is 2:1. It is a rodenticide used to control rats, squirrels, mice, moles, prairie dogs, ants and cockroaches. It is no longer registered for pesticide use in the United States. It has a role as a rodenticide and an insecticide. It is a thallium molecular entity and a metal sulfate. It contains a thallium(1+) and a sulfate.
General Description
Odorless white rhomboid prisms or a dense white powder. Density 6.77 g / cm3. Melting point 1170°F (632°C). Extremely toxic by ingestion. Very toxic by skin absorption and ingestion. A slow acting cumulative poison. Used as a rat poison, and an ant bait. Also used for analysis (testing for iodine in the presence of chlorine) and ozonometry. Not registered as a pesticide in the U.S.
Air & Water Reactions
Soluble in water.
Reactivity Profile
THALLIUM(I) SULFATE has weak oxidizing and weak reducing powers. Redox reactions can however still occur.
Health Hazard
Rated as extremely toxic. The probable oral lethal dose in humans is 5 to 50 mg/kg, or between 7 drops and 1 teaspoon for a 150-pound person. The mean lethal dose in an adult is probably about 1 gm of THALLIUM(I) SULFATE. Chronic exposure causes hair loss starting 10 days after exposure and complete baldness in about a month.
Fire Hazard
When heated to decomposition, THALLIUM(I) SULFATE emits very toxic fumes of thallium and sulfur oxide.
Safety Profile
Human poison by ingestion. Experimental poison by ingestion and subcutaneous routes. Human systemic effects by ingestion: ataxia, change in heart rate, excitement, eye changes, irritability, nausea or vomiting, nerve or sheath structural changes, somnolence, wakefulness. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of T1 and SOx. Used as a rat poison, ant bait, and a reagent in analytical chemistry. See also THALLIUM COMPOUNDS and SULFATES.
Carcinogenicity
Thallium is not classifiable with respect to carcinogenicity due to a lack of relevant human and animal studies. Several subchronic and chronic animal studies on thallium and compounds are available; however, they were not designed to examine carcinogenic end points.
Purification Methods
The sulfate crystallises from hot water (7mL/g) by cooling; then dry it under vacuum over P2O5. It is POISONOUS.
Properties of THALLIUM(I) SULFATE
| Melting point: | 632 °C(lit.) |
| Boiling point: | decomposes [STR93] |
| Density | 6.77 g/mL at 25 °C(lit.) |
| refractive index | 1.860 |
| storage temp. | Poison room |
| solubility | Water (Slightly) |
| form | Crystals or Powder |
| color | White to off-white |
| Specific Gravity | 6.77 |
| Water Solubility | Solubility in water increases with temperatureSoluble in water. |
| Merck | 14,9269 |
| CAS DataBase Reference | 7446-18-6(CAS DataBase Reference) |
| EPA Substance Registry System | Thallium(I) sulfate (7446-18-6) |
Safety information for THALLIUM(I) SULFATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H311:Acute toxicity,dermal H315:Skin corrosion/irritation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for THALLIUM(I) SULFATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
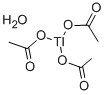

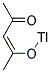

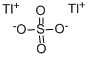
You may like
-
 Thallium (I) Sulphate CAS 7446-18-6View Details
Thallium (I) Sulphate CAS 7446-18-6View Details
7446-18-6 -
 Thallium(I) sulfate CAS 7446-18-6View Details
Thallium(I) sulfate CAS 7446-18-6View Details
7446-18-6 -
 Thallium(I) sulfate CAS 7446-18-6View Details
Thallium(I) sulfate CAS 7446-18-6View Details
7446-18-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8