THALLIUM(III) OXIDE
- CAS NO.:1314-32-5
- Empirical Formula: O3Tl2
- Molecular Weight: 456.76
- MDL number: MFCD00011276
- EINECS: 215-229-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57

What is THALLIUM(III) OXIDE?
Chemical properties
Thallium oxide is a solid, very dark brown and odourless chemical substance. Thallium oxide is stable under normal temperatures and pressures.
It is insoluble in water. Thallium oxide has been used to produce glasses with a high index of refraction.
The Uses of THALLIUM(III) OXIDE
Thallium(III) oxide is used in organic synthesis and as an analytical reagent
The Uses of THALLIUM(III) OXIDE
Analysis (testing for ozone), artificial gem, optical glass of high refractive index.
Production Methods
Thallium oxide is produced either by the chlorine-induced oxidation of thallous nitrate in an aqueous potassium hydroxide solution, followed by thallium reaction with oxygen, or from hydrogen peroxide and an alkaline thallium(I) solution ; or either form of thallous oxide oxidation. The oxide is an intermediate that can be formed during annealing or heating of semiconductors in air.
General Description
Brown powder. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A mixture of THALLIUM(III) OXIDE and antimony sulfide or sulfur explodes when ground in a mortar [Mellor 5:421 1946-47]. THALLIUM(III) OXIDE is decomposed by HCl with evolution of chlorine gas, and by H2SO4 with evolution of oxygen.
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Combustible by chemical reaction. Evolves O2 @ 875°C. Mixtures with sulfur orantimony trisulfide explode when ground. Hydrogen sulfide ignites and may explode weakly on contact with the oxide. When heated to decomposition it emits toxic fumes of T1. See also THALLIUM COMPOUNDS.
Properties of THALLIUM(III) OXIDE
| Melting point: | 717 °C(lit.) |
| Boiling point: | 875°C |
| Density | 9,65 g/cm3 |
| Flash point: | 875°C |
| solubility | insoluble in H2O; reacts with acid solutions |
| form | Powder |
| color | black |
| Water Solubility | Insoluble in water and alkalis. Decomposes in HCl and H2SO4 |
| Merck | 14,9268 |
| CAS DataBase Reference | 1314-32-5(CAS DataBase Reference) |
| EPA Substance Registry System | Thallium(III) oxide (1314-32-5) |
Safety information for THALLIUM(III) OXIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H330:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for THALLIUM(III) OXIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
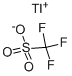
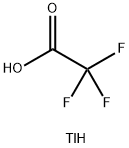
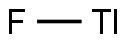
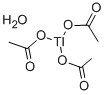


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8