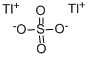CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Thallium sulfate appears as odorless white rhomboid prisms or a dense white powder. Density 6.77 g / cm3. Melting point 1170 °F (632 °C). Extremely toxic by ingestion. Very toxic by skin absorption and ingestion. A slow acting cumulative poison. Used as a rat poison, and an ant bait. Also used for analysis (testing for iodine in the presence of chlorine) and ozonometry. Not registered as a pesticide in the U.S. |
|---|---|
| Color/Form | White, rhomboid prisms |
| Odor | Odorless |
| Boiling Point | Decomposes (EPA, 1998) |
| Melting Point | 1170 °F (EPA, 1998) |
| Solubility | Solubility in 100 ml water at 0 °C: 2.70 g, at 20 °C: 4.87 g, at 100 °C: 18.45 g |
| Density | 6.77 (EPA, 1998) - Denser than water; will sink |
| Vapor Pressure | Inappreciable (EPA, 1998) |
| Stability/Shelf Life | Very stable |
| Corrosivity | Slightly corrosive to iron |
| Refractive Index | Index of refraction: 1.860, 1.867, 1.885 |
| Other Experimental Properties | Heat of fusion 5500 cal/g mole; 10.9 cal/g |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H311:Acute toxicity,dermal H315:Skin corrosion/irritation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 504.83 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 505.90058 g/mol |
| Monoisotopic Mass | 505.90058 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Thallium(I) sulfate is mainly used in organic synthesis as an oxidizing agent. It can facilitate the oxidation of various organic compounds, such as alcohols, to corresponding aldehydes or ketones. This reaction is commonly known as the Reformatsky reaction. Additionally, thallium sulfate is employed in the preparation of thallium salts for use in analytical chemistry and research applications. It is also utilized in the production of luminous paints, photoelectric cells, and low-melting glasses.