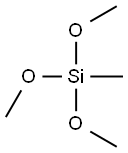
Tetramethyl orthosilicate
Synonym(s):Tetramethoxysilane;Tetramethyl orthosilicate
- CAS NO.:681-84-5
- Empirical Formula: C4H12O4Si
- Molecular Weight: 152.22
- MDL number: MFCD00008341
- EINECS: 211-656-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
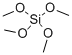
What is Tetramethyl orthosilicate?
Description
Tetramethyl orthosilicate is the chemical compound with the formula Si(OCH3)4. This molecule consists of four methoxy groups bonded to a silicon atom.
Two common organic precursors are tetraethyl orthosilicate (TEOS) and tetramethyl orthosilicate (TMOS). The latter is more toxic than the former.
Tetraethyl orthosilicate (TEOS), Si(OC2H5)4, is the first alkoxide of the series, followed by tetramethyl orthosilicate (TMOS), Si(OCH3)4, which is, however, less safe to handle and hydrolyzes faster than TEOS.
The hydrolysis of TMOS is, in fact, around six times faster: in general, a lower hydrolysis rate is associated with an increase of the organic group size in the silicon alkoxide. The properties of the silicon alkoxides change according to the dimension of the alkoxy; larger groups produce an increase in molecular weight, viscosity, and boiling point and a decrease in density of the alkoxides.
As a rule of thumb, a larger size of the alkoxy group is associated with a lower hydrolysis rate due to the steric hindrance. The reactivity follows the sequence, with tetramethyl orthosilicate the most reactive alkoxide:
tetramethyl orthosilicate >tetraethyl orthosilicate>tetra-n-propylorthosilicate>tetrabutyl orthosilicate
Chemical properties
Tetramethyl orthosilicate (TMOS), the methyl ester of orthosilicic acid, is a colorless, low-viscosity liquid. It is industrially the most important of the tetraalkyl silicates.
The Uses of Tetramethyl orthosilicate
Tetramethyl Orthosilicate is a compound used in the research of the multifunctionality of silicified nanoshells and the efficiency at adsorbing cadmium ions at cell interfaces.
The Uses of Tetramethyl orthosilicate
Tetramethyl orthosilicate (TMOS) is popularly used in the sol-gel synthesis of silicates1 and chromium-doped silicates and in the formation of hexagonal mesoporous silica layers.
The Uses of Tetramethyl orthosilicate
Coating screens of television picture tubes; mold binders; corrosion-resistant coatings; catalyst preparation; silicone intermediate
Production Methods
Silica aerogels are usually prepared by base-catalyzed reaction of tetramethoxysilane or tetraethoxysilane, mostly with ammonia as the catalyst. A modification of this procedure is to prehydrolyze Si(OR)4 with a small amount of water under acidic conditions.
General Description
A clear colorless liquid. Flash point below 125°F. Less dense than water and insoluble in water. Very toxic by ingestion and inhalation and very irritating to skin and eyes. Used to make paints and lacquers.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Tetramethyl orthosilicate is incompatible with the following: Oxidizers; hexafluorides of rhenium, molybdenum & tungsten .
Hazard
Eye damage and upper respiratory tract irri-tant.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Flammability and Explosibility
Flammable
Safety Profile
Poison by intraperitoneal route. Moderately toxic by inhalation. Midly toxic by skin contact. A severe eye irritant. This material can cause extensive necrosis (experimentally), keratoconus, and opaque cornea. It also causes severe human eye injuries, as well as necrosis of corneal cells, which progresses long after exposure has ceased. It is destructive and its effects resist treatment. Permanent blindness is possible from exposure to it. The kidney seems to be most subject to injury regardless of the mode of exposure. Pulmonary edema has also occurred. This material is more toxic than either ethyl silicate or silicic acid, although it has been thought that the injury caused is largely due to the action of the silicic acid. Flammable when exposed to heat or flame; can react vigorously with oxidizing materials. Potentially violent reaction with metal hexafluorides (e.g., rhenium, molybdenum, tungsten). When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Methyl silicate is used in coating screens of television picture tubes. It may be used in mold binders and in corrosion-resistant coatings; as well as in catalyst preparation and as a silicone intermediate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-heart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-First Aid: If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a cortico-steroid spray.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from water and moisture. Sources ofignition, such as smoking and open flames, are prohibitedwhere methyl silicate is used, handled, or stored in a manner that could create a potential fire or explosion hazard.
Shipping
UN2606 Methyl orthosilicate, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.
Purification Methods
Purification is as for tetraethoxysilane. It has a vapour pressure of 2.5mm at 0o. [IR: Sternbach & MacDiarmid J Am Chem Soc 81 5109 1959. Beilstein 1 IV 1266.]
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, including alkaline earth metals, metals, strong acids, strong bases; water, moisture, steam decomposes releasing toxic, flammable gases. Violent reaction with metal hexafluorides of rhenium, molybdenum, and tungsten. Contact with metals may evolve flammable hydrogen gas.
Properties of Tetramethyl orthosilicate
| Melting point: | −4 °C(lit.) |
| Boiling point: | 121-122 °C(lit.) |
| Density | 1.023 g/mL at 25 °C(lit.) |
| vapor density | 5.25 (vs air) |
| vapor pressure | 3.35 psi ( 20 °C) |
| refractive index | n |
| FEMA | 3185 | METHYLATED SILICA |
| Flash point: | 84 °F |
| storage temp. | Store below +30°C. |
| form | liquid |
| color | colorless |
| Specific Gravity | 1.032 |
| explosive limit | 0.88-23.8%(V) |
| Water Solubility | hydrolysis |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1699658 |
| CAS DataBase Reference | 681-84-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetramethyl silicate(681-84-5) |
| EPA Substance Registry System | Tetramethyl silicate (681-84-5) |
Safety information for Tetramethyl orthosilicate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tetramethyl orthosilicate
| InChIKey | LFQCEHFDDXELDD-UHFFFAOYSA-N |
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran

You may like
-
 681-84-5 / 12002-26-5 98%View Details
681-84-5 / 12002-26-5 98%View Details
681-84-5 / 12002-26-5 -
 Tetramethyl orthosilicate, 98% CAS 681-84-5View Details
Tetramethyl orthosilicate, 98% CAS 681-84-5View Details
681-84-5 -
 Tetramethoxysilane CAS 681-84-5View Details
Tetramethoxysilane CAS 681-84-5View Details
681-84-5 -
 Tetramethyl orthosilicate CAS 681-84-5View Details
Tetramethyl orthosilicate CAS 681-84-5View Details
681-84-5 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
