Tetrachloroethylene
Synonym(s):Ethylene tetrachloride, Perchloroethylene, PCE;PCE;Perchloroethylene;Tetrachloroethylene
- CAS NO.:127-18-4
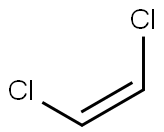
- Empirical Formula: C2Cl4
- Molecular Weight: 165.83
- MDL number: MFCD00000834
- EINECS: 204-825-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18

What is Tetrachloroethylene?
Description
Perchloroethylene (Tetrachloroethylene) is a colourless liquid with a slightly ethereal odour. It is marginally soluble in water and soluble in most organic solvents.Perchloroethylene has a limited number of uses and applications. It is used as intermediate, as dry cleaning agent in the industrial and professional sector, as surface cleaning agent in industrial settings, as heat transfer medium in industrial settings, and in film cleaning and copying by professionals. It is also used as a chemical intermediate in the production of fluorinated compounds and in industrial surface cleaning metal degreasing. Occupational exposure to perchloroethylene is possible in the manufacturing facilities or the industrial facilities where it is used as an intermediate.
Description
Tetrachloroethylene, often called perchloroethylene (PCE or “perc”), is a colorless, nonflammable liquid that is widely used for dry cleaning—which, of course, is not actually dry. It is?not?abbreviated as TCE because that is conventionally used for trichloroethylene.
In 1821, legendary chemist Michael Faraday discovered how to make PCE by heating hexachloroethane until it decomposed, with molecular chlorine as the byproduct. This process led to today’s wide variety of production methods in which light hydrocarbons or chlorohydrocarbons are heated in the presence of chlorine, with or without a catalyst, to yield a combination of PCE and many other chlorocarbons. The crude mixture must be separated by distillation
In addition to dry cleaning, PCE is used industrially as a solvent, degreaser, refrigerant, and starting material for fluorocarbon production. Its use in dry cleaning?has declined precipitously in the past 40 years?because of improved solvent recycling systems and worker health concerns (it is a probable carcinogen). Despite this downturn, its overall worldwide production is slowly increasing.
Potential PCE replacements for dry cleaning include 1-bromopropane, silicone fluids, and liquified carbon dioxide, all of which have health, environmental, or economic drawbacks. So when you send your holiday finery out for cleaning, it’s most likely that it will be treated with PCE.
Chemical properties
Tetrachloroethylene is a clear, colorless, nonflammable liquid with a characteristic odor. The odor is noticeable @ 47 ppm, though after a short period it may become inconspicuous, thereby becoming an unreliable warning signal. The Odor Threshold is variously given as 5 ppm to 6.17 (3M).
Physical properties
Clear, colorless, nonflammable liquid with a chloroform or sweet, ethereal odor. Odor threshold concentration is 4.68 ppmv (Leonardos et al., 1969). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.24 and 2.8 mg/L, respectively (Alexander et al., 1982).
The Uses of Tetrachloroethylene
Tetrachloroethylene is used as a solvent, indrycleaning, and in metal degreasing.Tetrachloroethylene is a common industrial solvent that is often found as a contaminant in groundwater. Tetrachloroethylene is also a suspected carcinogen to humans and is difficult to degrade biologically as it has no natural source. This compound is a contaminant of emerging concern (CECs).
Definition
ChEBI: A chlorocarbon that is tetrachloro substituted ethene.
Production Methods
Tetrachloroethylene (PCE) was first prepared in 1821 and commercial production in the United States began in 1925. Several commercial grades are available that differ in the amount and type of added stabilizers (e.g., amines, phenols, and epoxides).
The industrial processes for production of tetrachloroethylene include threetechnical routes:
(1) chlorination of trichloroethylene and followed by dehydrochlorination.
(2) oxychlorination of ethylene.
(3) chlorination andpyrolysis of light hydrocarbon.
In China, the chlorination of trichloroethylene and dehydrochlorination process is mainly used for production of tetrachloroethylene, while the other two processes are widely used in other countries.
Synthesis Reference(s)
Journal of the American Chemical Society, 90, p. 5307, 1968 DOI: 10.1021/ja01021a065
General Description
Tetrachloroethylene (perchloroethylene, PCE), is a chlorinated ethylene compound commonly used as a dry cleaning and degreasing solvent. It shows IR transparency as it has no C–H bonds making it an ideal solvent for IR spectroscopy. PCE is a man-made pollutant which is difficult to degrade. It is a ground water contaminant which has adverse effect on human health due to its potential toxicity and carcinogenicity. Some of the methods proposed for its degradation are Fenton oxidation treatment, reductive dehalogenation under methanogenic condition, and reduction using zero valent metal ions. One of the methods reported for its synthesis is from ethylene dichloride and chlorine.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Tetrachloroethylene decomposes upon heating and exposure to UV light to give phosgene and HCl. Reacts violently with finely dispersed light metals (aluminum) and zinc. [Handling Chemicals Safely 1980 p. 887]. Mixtures with finely divided barium or lithium metal can detonate [ASESB Pot. Incid. 39. 1968; Chem. Eng. News 46(9):38. 1968]. Decomposes very slowly in water to form trichloroacetic acid and hydrochloric acid
Health Hazard
Exposure to tetrachloroethylene can produceheadache, dizziness, drowsiness, incoordina tion, irritation of eyes, nose, and throat, and flushing of neck and face. Exposure to highconcentrations can produce narcotic effects.The primary target organs are the centralnervous system, mucous membranes, eyes,and skin. The kidneys, liver, and lungs areaffected to a lesser extent. Symptoms ofdepression of the central nervous system aremanifested in humans from repeated expo sure to 200 ppm for 7 hours/day. Chronicexposure to concentrations ranging from 200to 1600 ppm caused drowsiness, depression,and enlargement of the kidneys and livers inrats and guinea pigs. A 4-hour exposure to4000 ppm of vapor in air was lethal to rats.
Ingestion of tetrachloroethylene may pro duce toxic effects ranging from nausea andvomiting to somnolence, tremor, and ataxia.The oral toxicity, is low, however, with LD50ranging between 3000 and 9000 mg/kg inanimals. Skin contact with the liquid maycause defatting and dermatitis of skin.
Evidence of carcinogenicity of this com pound has been noted in test animals sub jected to inhalation or oral administration. Itcaused tumors in the blood, liver, and kidneyin rats and mice. Carcinogenicity in humansis not reported.
Fire Hazard
Special Hazards of Combustion Products: Toxic, irritating gases may be generated in fires.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Animal carcinogen that produces increased incidence of renal adenomas, adenocarcinomas, mononuclear cell leukemia, and hepatocellular tumors.
Safety Profile
Tetrachloroethylene is a nonflammable colorless liquid with a sharp sweet odor. Tetrachloroethylene is widely used for dry-cleaning fabrics and metal degreasing operations. Effects resulting from acute (short term) high-level inhalation exposure of humans to tetrachloroethylene include irritation of the upper respiratory tract and eyes, kidney dysfunction, and neurological effects such as reversible mood and behavioral changes, impairment of coordination, dizziness, headache, sleepiness, and unconsciousness. The primary effects from chronic (long term) inhalation exposure are neurological, including impaired cognitive and motor neurobehavioral performance. Tetrachloroethylene exposure may also cause adverse effects in the kidney, liver, immune system and hematologic system, and on development and reproduction. Studies of people exposed in the workplace have found associations with several types of cancer including bladder cancer, non-Hodgkin lymphoma, multiple myeloma. U.S. EPA has classified tetrachloroethylene as likely to be carcinogenic to humans.
Potential Exposure
Tetrachloroethylene is used in the textile industry and as a chemical intermediate or a heatexchange fluid; a widely used solvent with particular use as a dry cleaning agent; a degreaser; a fumigant, and medically as an anthelmintic.
Carcinogenicity
Tetrachloroethylene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN1897 Tetrachloroethylene, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
It decomposes under similar conditions to CHCl3, to give phosgene and trichloroacetic acid. Inhibitors of this reaction include EtOH, diethyl ether and thymol (effective at 2-5ppm). Tetrachloroethylene should be distilled under a vacuum (to avoid phosgene formation) and stored in the dark out of contact with air. It can be purified by washing with 2M HCl until the aqueous phase no longer becomes coloured, then with water, drying with Na2CO3, Na2SO4, CaCl2 or P2O5, and fractionally distilling just before use. 1,1,2-Trichloroethane and 1,1,1,2-tetrachloroethane can be removed by counter-current extraction with EtOH/water. [Beilstein 1 IV 715.]
Incompatibilities
Violent reaction with strong oxidizers; powdered, chemically active metals, such as aluminum, lithium, beryllium, and barium; caustic soda; sodium hydroxide; potash. Tetrachloroethylene is quite stable. However, it reacts violently with concentrated nitric acid to give carbon dioxide as a primary product. Slowly decomposes on contact with moisture producing trichloroacetic acid and hydrochloric acid. Decomposes in UV light and in temperatures above 150℃, forming hydrochloric acid and phosgene.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Alternatively, PCE may be recovered from waste gases and reused.
Properties of Tetrachloroethylene
| Melting point: | -22 °C (lit.) |
| Boiling point: | 121 °C (lit.) |
| Density | 1.623 g/mL at 25 °C (lit.) |
| vapor density | 5.83 (vs air) |
| vapor pressure | 13 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 120-121°C |
| storage temp. | Store at +2°C to +25°C. |
| solubility | water: soluble0.15g/L at 25°C |
| form | Liquid |
| appearance | colorless liquid |
| color | APHA: ≤10 |
| Odor | chloroform-like odor |
| Odor Threshold | 0.77ppm |
| Water Solubility | Miscible with alcohol, ether, chloroform, benzene and hexane. Slightly miscible with water. |
| FreezingPoint | -22.0℃ |
| λmax | λ: 290 nm Amax: 1.00 λ: 295 nm Amax: 0.30 λ: 300 nm Amax: ≤0.20 λ: 305 nm Amax: 0.10 λ: 350 nm Amax: 0.05 λ: 400 nm Amax: 0.03 |
| Merck | 14,9190 |
| BRN | 1361721 |
| Henry's Law Constant | 4.97 at 1.8 °C, 15.5 at 21.6 °C, 34.2 at 40.0 °C, 47.0 at 50 °C, 68.9 at 60 °C, 117.0 at 70 °C
(EPICS-GC, Shimotori and Arnold, 2003) |
| Exposure limits | TLV-TWA 50 ppm (~325 mg/m3) (ACGIH),
100 ppm (MSHA and OSHA); TLV-STEL
200 ppm (ACGIH); carcinogenicity: Animal
Limited Evidence. |
| Dielectric constant | 2.5(21℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, alkali metals, aluminium, strong bases. |
| CAS DataBase Reference | 127-18-4(CAS DataBase Reference) |
| IARC | 2A (Vol. Sup 7, 63, 106) 2014 |
| NIST Chemistry Reference | Tetrachloroethylene(127-18-4) |
| EPA Substance Registry System | Tetrachloroethylene (127-18-4) |
Safety information for Tetrachloroethylene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tetrachloroethylene
Tetrachloroethylene manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 PERCHLOROETHYLENE (PCE) 99%View Details
PERCHLOROETHYLENE (PCE) 99%View Details -
 Perchloroethylene 99%View Details
Perchloroethylene 99%View Details -
 Perchloro Ethylene 99%View Details
Perchloro Ethylene 99%View Details -
 Tetrachloroethylene 99%View Details
Tetrachloroethylene 99%View Details -
 Tetrachloroethylene, Ultrapure CAS 127-18-4View Details
Tetrachloroethylene, Ultrapure CAS 127-18-4View Details
127-18-4 -
 Tetrachloroethylene, Ultrapure CAS 127-18-4View Details
Tetrachloroethylene, Ultrapure CAS 127-18-4View Details
127-18-4 -
 Tetrachloroethylene, Ultrapure CAS 127-18-4View Details
Tetrachloroethylene, Ultrapure CAS 127-18-4View Details
127-18-4 -
 Tetrachloroethylene pure CAS 127-18-4View Details
Tetrachloroethylene pure CAS 127-18-4View Details
127-18-4
