CIS-1,2-DICHLOROETHYLENE
Synonym(s):cis-1,2-DCE;cis-1,2-Dichloroethylene;cis-Acetylene dichloride
- CAS NO.:156-59-2
- Empirical Formula: C2H2Cl2
- Molecular Weight: 96.94
- MDL number: MFCD00000925
- EINECS: 205-859-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
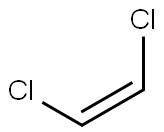
What is CIS-1,2-DICHLOROETHYLENE?
Chemical properties
clear colorless liquid
Chemical properties
1,2-Dichloroethylene exists in three isomers, sym-, cis-60% and trans-40%. There are variations in toxicity between these two forms. At room temperature, these chemicals are colorless liquids with a slightly acrid, ethereal odor. The Odor Threshold in air is 17 ppm. sym-isomer:
The Uses of CIS-1,2-DICHLOROETHYLENE
cis-1,2-Dichloroethylene (cis-1,2-DCE) is generally used in the preparation of various enediynes for Bergman cyclization. Applications include the synthesis of:
- 2-(6-substituted 3(Z)-hexen-1,5-diynyl)anilines to prepare corresponding substituted carbazoles.
- (Z)-1-aryl-3-henen-1,5-diynes to obtain the corresponding aryl substituted benzotriazoles.
It is used as a linker to synthesize boron-dipyrromethene (BODIPY)-based photosensitizer for photodynamic therapy (PDT). cis-1,2-DCE can also be used as a precursor to synthesize intermediates of sporolide B.
Definition
ChEBI: Cis-1,2-dichloroethene is a 1,2-dichloroethene.
General Description
A clear colorless liquid with an ether-like odor. Flash point 36-39°F. Denser than water and insoluble in water. Vapors heavier than air. Used in the making of perfumes.
Air & Water Reactions
Highly flammable.Slightly soluble in water.
Reactivity Profile
1,2-DICHLOROETHYLENE and potassium hydroxide forms chloroacetylene, which is explosive and spontaneously flammable in air. CIS-1,2-DICHLOROETHYLENE is highly toxic, Rutledge, p134(1968).
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. May polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Mildly toxic by ingestion and inhalation. In high concentration it is irritating and narcotic. Has produced liver and kidney injury in experimental animals. Mutation data reported. Sometimes thought to be nonflammable, however, it is a dangerous fire hazard when exposed to heat or flame. Reaction with solid caustic alkalies or their concentrated solutions produces chloracetylene gas, whch ignites spontaneously in air. Reacts violently with N2O4, KOH, Na, NaOH. Moderate explosion hazard in the form of vapor when exposed to flame. Can react vigorously with oxidizing materials. To fight fire, use water spray, foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also VINYLIDENE CHLORIDE and CHLORINATED HYDROCARBONS, ALIPHATIC.
Potential Exposure
Primary irritant (w/o allergic reaction). 1,2-Dichloroethylene is used as a solvent for waxes, resins, and acetylcellulose. It is also used in the extraction of rubber, as a refrigerant; in the manufacture of pharmaceuticals and artificial pearls; and in the extraction of oils and fats from fish and meat.
Shipping
UN1150 Dichloroethylene, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Purify it by careful fractional distillation, followed by passage through neutral activated alumina. Also by shaking with mercury, drying with K2CO3 and distilling from CaSO4. Stabilise it with 0.02% of 2,6-di-tert-butyl-p-cresol. [Beilstein 1 IV 707.]
Incompatibilities
May form explosive mixture with air. Attacks some plastics, rubber, and coatings. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Gradual decomposition results in hydrochloric acid formation in the presence of ultraviolet light or upon contact with hot metal or other hot surfaces. Reacts with strong bases; potassium hydroxide; difluoromethylene, dihypofluoride, nitrogen tetroxide (explosive); or copper (and its alloys) producing toxic chloroacetylene which is spontaneously flammable on contact with air. Attacks some plastics and coatings.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of CIS-1,2-DICHLOROETHYLENE
| Melting point: | -80 °C (lit.) |
| Boiling point: | 60 °C (lit.) |
| Density | 1.284 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 43 °F |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | 3.5g/L(25 ºC) |
| Merck | 14,92 |
| BRN | 1071208 |
| Dielectric constant | 9.1999999999999993 |
| Stability: | Volatile |
| CAS DataBase Reference | 156-59-2(CAS DataBase Reference) |
| EPA Substance Registry System | cis-1,2-Dichloroethylene (156-59-2) |
Safety information for CIS-1,2-DICHLOROETHYLENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for CIS-1,2-DICHLOROETHYLENE
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran

You may like
-
 cis-1,2-Dichloroethylene CAS 156-59-2View Details
cis-1,2-Dichloroethylene CAS 156-59-2View Details
156-59-2 -
 cis-1,2-Dichloroethene solution CAS 156-59-2View Details
cis-1,2-Dichloroethene solution CAS 156-59-2View Details
156-59-2 -
 cis-1,2-Dichloroethene CAS 156-59-2View Details
cis-1,2-Dichloroethene CAS 156-59-2View Details
156-59-2 -
 cis-Dichloroethylene CAS 156-59-2View Details
cis-Dichloroethylene CAS 156-59-2View Details
156-59-2 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
