Testolactone
- CAS NO.:968-93-4
- Empirical Formula: C19H24O3
- Molecular Weight: 300.39
- MDL number: MFCD00866295
- EINECS: 213-534-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-11-01 18:37:15
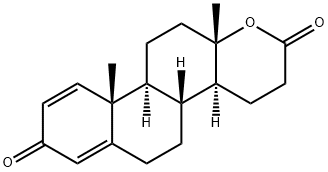
What is Testolactone?
Absorption
Testolactone is well absorbed from the gastrointestinal tract.
Toxicity
Oral LD50s in mouse and dog are 1630 mg/kg and 593-926 mg/kg, respectively.
Originator
Fludestrin,Heyden,W. Germany,1968
The Uses of Testolactone
Testolactone USP (Teslac) is used to treat Breast cancer.
The Uses of Testolactone
Antineoplastic; non-selective steroidal aromatase inhibitor that prevents the conversion from androgen to estrogen. Used in in the treatment of familial male precocious puberty. An effective anti-tumor agent.
Background
An antineoplastic agent that is a derivative of progesterone and used to treat advanced breast cancer.
Indications
For palliative treatment of advanced breast cancer in postmenopausal women.
Definition
ChEBI: Testolactone is a seco-androstane and a 3-oxo-Delta(1),Delta(4)-steroid.
Manufacturing Process
(a) Fermentation: A medium of the following composition is prepared: 3.0
grams cornsteep liquor solids; 3.0 grams NH4H2PO4; 2.5 grams CaCO3; 2.2
grams soybean oil; 0.5 gram progesterone and distilled water to make 1 liter.
The medium is adjusted to pH 7.00.1. Then, 100 ml portions of the medium
are distributed in 500 ml Erlenmeyer flasks and the flasks plugged with cotton
and sterilized in the usual manner (i.e., by autoclaving for 30 minutes at
120°C). When cool, each of the flasks is inoculated with 5 to 10% of a
vegetative inoculum of Cylindrocarpon radicola [the vegetative inoculum being
grown from stock cultures (lyophilized vial or agar slant) for 48 to 72 hours in
a medium of the following composition: 15 grams cornsteep liquor; 10 grams
brown sugar; 6 grams NaNO3; 0.001 gram ZnSO4; 1.5 grams KH2PO4;0.5
gram MgSO47H2O; 5 grams CaCO3; 2 grams lard oil; and distilled water to
make 1 liter].
The flasks are then placed on a reciprocating shaker (120 one and one-half
inch cycles per minute) and mechanically shaken at 25°C for 3 days. The
contents of the flasks are then pooled and, after the pH of the culture is
adjusted to about 40.2 with sulfuric acid, filtered through Seitz filter pads to
separate the mycelium from the fermented medium.
(b) Extraction: 40 liters of the culture filtrate obtained in (a) is extracted with
40 liters chloroform in an extractor (e.g., Podbelniak, US Patent 2,530,886, or
improvements thereon) and the filtered chloroform extract is evaporated to
dryness in vacuo. The residue (11.1 grams) is taken up in 200 ml of 80%
aqueous methanol, and the resulting solution is extracted four times with 100
ml portions of hexane. The 80% aqueous methanol solution is then
concentrated in vacuo until crystals appear; and, after cooling at 0°C for
several (usually about 3 to 4) hours, the crystals formed are recovered by
filtration. About 2.9 grams 1-dehydrotestololactone (MP 217° to 217.5°C) are
thus obtained. Concentration of the mother liquors yields additionally about
6.0 grams of the lactone. Recrystallization from acetone yields a purified 1-dehydrotestololactone having a melting point of 218° to 219°C.
Therapeutic Function
Cancer chemotherapy
General Description
Testolactone, 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acidΔ-lactone (Teslac),was originally synthesized as a possible anabolic steroid,considering its structural similarity to testosterone. The keystructural difference from anabolic steroids is the D-ring lactone instead of the typical cyclopentyl ring. Althoughconsidered in many texts an androgen or anabolic steroid (itis a Schedule III drug because of its classification as an anabolicsteroid), testolactone lacks androgenic effects in vivo.Its action is believed to be caused by irreversible inhibitionof aromatase. It is a relatively weak inhibitor of aromatase,but the irreversible nature of the inhibition can lead to prolongedeffects. Its relatively weak inhibition of aromataseand its undesirable dosage schedule (5×50-mg tabletsq.i.d.) give this older agent only limited use in breast cancertreatment because of better available options.
Pharmacokinetics
Testolactone is a synthetic anti-neoplastic agent that is structurally distinct from the androgen steroid nucleus in possessing a six-membered lactone ring in place of the usual five-membered carbocyclic D-ring. Despite some similarity to testosterone, testolactone has no in vivo androgenic effect. No other hormonal effects have been reported in clinical studies in patients receiving testolactone.
Metabolism
Hepatic. Metabolized to several derivatives in the liver, all of which preserve the lactone D-ring.
Properties of Testolactone
| Melting point: | 218-219° |
| Boiling point: | 482.0±45.0 °C(Predicted) |
| alpha | D23 -45.6° (c = 1.24 in chloroform) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 968-93-4(CAS DataBase Reference) |
Safety information for Testolactone
Computed Descriptors for Testolactone
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran



You may like
-
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Phenylephrine HCl 61-76-7 98%View Details
Phenylephrine HCl 61-76-7 98%View Details
61-76-7 -
 211915-06-9 98%View Details
211915-06-9 98%View Details
211915-06-9 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 201530-41-8 Deferasirox 98%View Details
201530-41-8 Deferasirox 98%View Details
201530-41-8 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
