Spironolactone
Synonym(s):4-Pregnen-21-oic acid-17α-ol-3-one-7α-thiol γ-lactone 7-acetate;7α-(Acetylthio)-17α-hydroxy-3-oxopregn-4-ene-21-carboxylic acid γ-lactone;Spironolactone
- CAS NO.:52-01-7
- Empirical Formula: C24H32O4S
- Molecular Weight: 416.58
- MDL number: MFCD00082250
- EINECS: 200-133-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:42
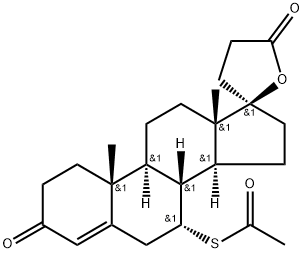
What is Spironolactone?
Absorption
The mean time to reach peak plasma concentration of spironolactone and the active metabolite, canrenone, in healthy volunteers is 2.6 and 4.3 hours, respectively. Food increased the bioavailability of spironolactone (as measured by AUC) by approximately 95.4%.
Toxicity
The oral LD50 of spironolactone is greater than 1000 mg/kg in mice, rats, and rabbits.
Acute overdosage of ALDACTONE may be manifested by drowsiness, mental confusion, maculopapular or erythematous rash, nausea, vomiting, dizziness, or diarrhea. Rarely, instances of hyponatremia, hyperkalemia,or hepatic coma may occur in patients with severe liver disease, but these are unlikely due to acute overdosage. Hyperkalemia may occur, especially in patients with impaired renal function. In case of an overdose, vomiting may be induced and gastric lavage may be instituted. As there is no specific antidote, treatment is supportive to maintain hydration, electrolyte balance, and vital functions. Patients who have renal impairment may develop hyperkalemia. In such cases, discontinue spironolactone.
Description
Spironolactone is a steroidal aldosterone blocker that was first reported in 1959 by John A. Cella and Robert C. Tweit at G. D. Searle & Co.1 (Skokie, IL). Aldosterone is an essential corticosteroid hormone that conserves sodium in the kidneys and other organs and regulates blood pressure. But it must be suppressed to treat fluid buildup in patients with heart failure, high blood pressure, and other conditions.
Cella and Tweit’s article was the second in a series on novel aldosterone blockers. In the first article, they reported compounds that worked best when administered parenterally. Spironolactone and similar compounds showed improved blocking activity when administered orally.
Spironolactone is on the World Health Organization''s List of Essential Medicines. To this day, it is prescribed more than ten million times a year in the United States. The drug, however, has numerous adverse side effects, including high blood potassium, nausea, frequent urination, and dehydration. It also has estrogen-like effects, which can decrease sex drive in men. In recent years, this estrogenic property has been useful in feminizing hormone therapy for transgender women.
1. Searle was acquired by Monsanto in 1985 and was eventually absorbed into Pfizer.
Chemical properties
White to Off White Solid
Originator
Aldactone,Searle,US,1959
The Uses of Spironolactone
Spironolactone, an aldosterone-, and competitive androgen-receptor-antagonist and 5-alpharductase- inhibitor, indicated for the treatment of androgen dependent hirsutism, ideally in doses of 50 to 200 mg per day accompanying the intake of oral contraceptives with the same seven day break in between. Side effects concerning the length of the menstrual cycle, the increase of blood pressure or potassium levels may occur. Spironolactone is the number one drug in the treatment of hirsutism in the US (Farquhar et al., 2003). In other countries the prescription of spironolactone for the treatment of hirsutism may be off-label.
The Uses of Spironolactone
It is a synthetic 17-lactone steroid which is a renal competitive aldosterone antagonist in a class of pharmaceuticals called potassium-sparing diuretics, used primarily to treat ascites in patients with liver disease, low-renin hypertension, hypokalemia
The Uses of Spironolactone
diuretic, aldosterone antagonist
What are the applications of Application
Spironolactone is a lactone for proteomic research
Indications
Spironolactone is indicated for the treatment of the following conditions:
As spironolactone has antiandrogenic activity, its off-label uses include the treatment of hirsutism, female pattern hair loss, and adult acne vulgaris.
Background
Spironolactone is a potassium-sparing diuretic. It binds to mineralocorticoid receptors and functions as aldosterone antagonists. It promotes sodium and water excretion and potassium retention. Spironolactone was originally developed purely for this ability before other pharmacodynamic properties of the drug were discovered. It is indicated to treat several conditions, including heart failure, edema, hyperaldosteronism, and hypertension. Off-label uses of spironolactone include hirsutism, female pattern hair loss, and adult acne vulgaris.
Spironolactone was developed in 1957, marketed in 1959, and approved by the FDA on January 21, 1960.
Definition
ChEBI: A steroid lactone that is 17alpha-pregn-4-ene-21,17-carbolactone substituted by an oxo group at position 3 and an alpha-acetylsulfanyl group at position 7.
Indications
Spironolactone (Aldactone) is a compound originally developed as a mineralocorticoid antagonist and is used as a diuretic and antihypertensive agent. However, at high doses spironolactone binds to the androgen receptor. In clinical practice it is a weak androgen antagonist used to treat hirsutism in women by blocking testosterone binding to androgen receptors in hair follicles. Use of spironolactone in women for the treatment of hirsutism or male pattern baldness can result in elevated serum potassium levels; these levels should be checked within 1 month of starting the medication.
Manufacturing Process
A mixture of approximately 11 parts of 17α-(2-carboxyethyl)-17β- hydroxyandrosta-4,6-dien-3-one lactone and 10 parts of thioacetic acid is heated at 85° to 95°C for ? hour. Excess thioacetic acid is removed by vacuum distillation at this point, and the residue is twice recrystallized from methanol, affording 7α-acetylthio-17α-(2-carboxyethyl)-17β-hydroxyandrost- 4-en-3-one lactone, melting at approximately 134° to 135°C. Heated above this melting point, the product solidifies and melts again at approximately 201° to 202°C (with decomposition).
brand name
Aldactone (Searle);Airolactone;Aldactide 25;Aldactone-a;Aldazida;Aldonorm;Aldospirone;Alpamed;Altexide;Aporasnon;Carditan;Crk 635;Ct-spiro;Digi-aldopur;Dilakton;Hexalacton;Hokulaton;Hokuraton;Hydrospiron;Idrolattone;Lacilactone;Laralmin;Lasitone;Loractone;Mf 218d;Noidouble;Novospiroton;Novospirozine;Novosprioton;Novospriozine;Penantin;Pirolacton;Pirolcaton;Plarenil;Practon 50;Raudazida;Risicordin;Rolactone microfine;Sali-spiroctan;Sas 1060;Servilactone;Spiractin;Spiridazide;Spirix;Spiro comp;Spiro50-d;Spirodigital;Spiro-f;Spironomocompren;Spironone;Spironothiazide;Spiropal;Spirostada;Spirotone;Suprapuren;Synureticum;Tensoflex;Urosonine;Xenalone;Xeualon.
Therapeutic Function
Diuretic
World Health Organization (WHO)
Spironolactone, an aldosterone antagonist, has been widely used for over 25 years in the treatment of hypertension and in the management of refractive oedema. Evidence that long-term administration of high doses are tumorigenic in the rat has recently led to restriction of its use by some national regulatory authorities although the significance of this finding with respect to clinical use is not certain. In 1987 spironolactone was transferred from the main list to the complementary list of the WHO Model List of Essential Drugs. (See also WHO comments for canrenone and potassium canrenoate). (Reference: (WHODI) WHO Drug Information, 2(1), , 1988)
Biological Functions
Spironolactone (Aldactone) is structurally related to aldosterone and acts as a competitive inhibitor to prevent the binding of aldosterone to its specific cellular binding protein. Spironolactone thus blocks the hormone-induced stimulation of protein synthesis necessary for Na+ reabsorption and K+ secretion. Spironolactone, in the presence of circulating aldosterone, promotes a modest increase in Na+ excretion associated with a decrease in K+ elimination. The observations that spironolactone is ineffective in adrenalectomized patients and that the actions of spironolactone can be reversed by raising circulating al-dosterone blood levels (surmountable antagonism) support the conclusion that spironolactone acts by competitive inhibition of the binding of aldosterone with receptor sites in the target tissue. Spironolactone acts only when mineralocorticoids are present.
General Description
Spironolactone, 7α-(acetylthio)-17α-hydroxy-3-oxopregn-4-ene-3-one-21-carboxylic acidγ-lactone (Aldactone) is an aldosterone antagonist of greatmedical importance because of its diuretic activity.
Hazard
Questionable carcinogen.
Biological Activity
Competitive mineralocorticoid (aldosterone) receptor antagonist that exhibits antihypertensive activity in vivo . Also displays antiandrogen activity and inhibits steroid hormone biosynthesis.
Biochem/physiol Actions
Spironolactone is a competitive aldosterone receptor antagonist. Used as potassium sparing diuretic.
Mechanism of action
Spironolactone is a potassium sparing diuretic that has a different mechanism of action than other drugs of this class. It is a competitive antagonist of aldosterone, and its action is most effective when the level of circulated aldosterone in the organism is high.
Pharmacokinetics
Spironolactone has a potassium-sparing diuretic effect. It promotes sodium and water excretion and potassium retention. It increases renin and aldosterone levels. Spironolactone is a mineralocorticoid receptor antagonist and has a low affinity for the glucocorticoid receptor. It also exhibits progestogenic and anti-androgenic actions as it binds to the androgen receptor and, to a lesser extent, estrogen and progesterone receptors. Spironolactone exhibits anti-inflammatory effects.
Pharmacology
Spironolactone (Aldactone) is the only diuretic that has
been shown in a double-blind multicenter prospective
clinical trial to improve survival in CHF. The addition
of spironolactone to digitalis and an angiotensinconverting
enzyme (ACE) inhibitor significantly improved
survival among patients with chronic severe
heart failure.
Spironolactone competitively inhibits the binding of
aldosterone to cytosolic mineralocorticoid receptors in
the epithelial cells in the late distal tubule and collecting duct of the kidney. Aldosterone enhances salt and
water retention at the expense of enhanced renal K
and H excretion. Spironolactone enhances diuresis by
blocking sodium and water retention while retaining
potassium. An obvious potential side effect is hyperkalemia,
which is aggravated by the potassium-retaining
properties of the ACE inhibitors. The likely concomitant
use of the loop diuretic furosemide, which depletes
K , dictates careful monitoring of serum potassium to
avoid life-threatening rhythm disturbances.
There is also evidence for the existence of mineralocorticoid
receptors on cardiac myocytes. This raises the
intriguing possibility that spironolactone could mediate
important direct effects on the myocardium in CHF.
Clinical Use
Spironolactone has been used clinically in the following
conditions:
1. Primary hyperaldosteronism. Used as an aid in
preparing patients with adrenal cortical tumors
for surgery.
2. Hypokalemia. Used in patients with low serum K+
resulting from diuretic therapy with other agents.
Its use should be restricted to patients who are unable
to supplement their dietary K+ intake or adequately
restrict their salt intake or who cannot
tolerate orally available KCl preparations.
3. Hypertension and congestive heart failure.
Although spironolactone may be useful in combination
with thiazides, the latter remain the
drugs of first choice. Fixed-dose combinations of
spironolactone and a particular thiazide (e.g.,
Aldactazide) generally offer no therapeutic advantage
over either component given separately
and tend to restrict the ability of the clinician to
determine the optimal dosage of each drug for a
particular patient.
4. Cirrhosis and nephrotic syndrome. Spironolactone
is a mild diuretic and may be useful in treating the
edema that occurs in these two clinical conditions,
that is, when excessive K+ loss is to be avoided.
Side Effects
Serum electrolyte balance should be monitored periodically, since potentially fatal hyperkalemia may occur,especially in patients with impaired renal function or excessive K+ intake (including the K+ salts of coadministered drugs, e.g., potassium penicillin). Spironolactone can induce hyponatremia and in cirrhotic patients, metabolic acidosis.A variety of gastrointestinal disturbances may accompany spironolactone administration. These include diarrhea, gastritis, gastric bleeding, and peptic ulcers. Spironolactone is contraindicated in patients with peptic ulcers. Spironolactone may also cause elevated blood urea nitrogen, drowsiness, lethargy, ataxia, confusion, and headache. Gynecomastia and menstrual irregularity in males and females, respectively, can occur. Painful gynecomastia (directly related to dosage level and duration of therapy), which is generally reversible, may necessitate termination of therapy. Animal studies demonstrating tumorigenic potential support the clinical judgment that spironolactone alone or in combination should not be used for most patients who require diuretic therapy and its unnecessary use should be avoided.
Safety Profile
Poison by intraperitoneal route. Human reproductive effects by ingestion and possibly other routes: men, impotence and breast development; women, menstrual cycle changes or disorders, changes in the breasts and lactation. An experimental teratogen. Other experimental reproductive effects. Other human systemic effects by ingestion: agranulocytosis, kidney tubule damage, increased urine volume, and changes in blood sodium and calcium levels. Questionable carcinogen. When heated to decomposition it emits toxic fumes of SOx,. Used to treat hypertension, edema of congestive heart failure, cirrhosis, and kidney failure. 0
Synthesis
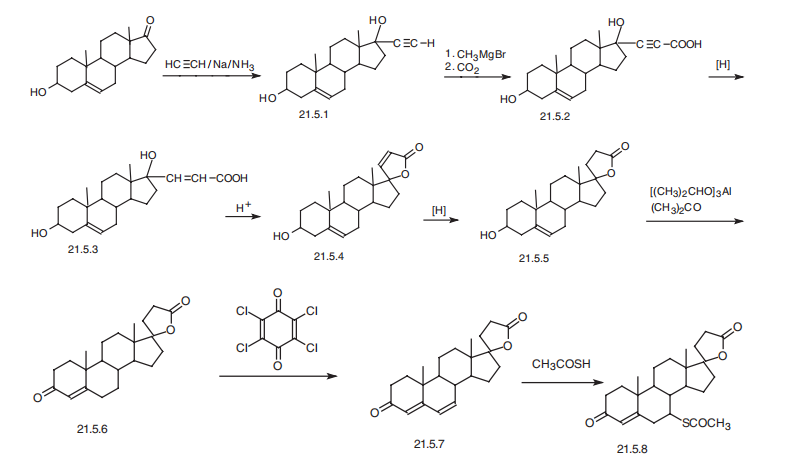
Spironolactone is the 7-acetate of the |?-lactone of 17-hydroxy-7-mercapto- 3-oxo-17-|á-pregn-4-ene-21-carboxylic acid (21.5.8). Spironolactone is synthesized industrially in two different ways from androstenolone?a3|?-hydroxy-5-androsten-17-one. According to the first method, androstenolone undergoes ethynylation by acetylene in a Normant reaction condition using sodium amide in liquid ammonia, which forms 17 |á-ethynyl-3|?-,17|?-dihydroxy-5-androstene (21.5.1). Subsequent reaction of this with methylmagnesiumbromide and then with carbon dioxide gives the corresponding propenal acid (21.5.2). Reduction of the triple bond in this product with hydrogen using a palladium on calcium carbonate catalyst forms the corresponding acrylic acid derivative (21.5.3), which is treated with acid without being isolated, which leads to cyclization into an unsaturated lactone derivative (21.5.4). The double bond is reduced by hydrogen, in this case using a palladium on carbon catalyst. The resulting lactone 21.5.5 undergoes oxidation in an Oppenauer reaction, giving an unsaturated keto-derivative?a4-androsten-3,17-dione (21.5.6). Further oxidation of the product (21.5.6) using chloroanyl gives dienone (21.5.7), which when reacted with thioacetic acid gives the desired spionolactone (21.5.8).
Veterinary Drugs and Treatments
Spironolactone may be used in patients with congestive heart failure who do not adequately respond to furosemide and ACE inhibitors, who develop hypokalemia on other diuretics, and are unwilling or unable to supplement with exogenous potassium sources. It may also be effective in treating ascites as it has less potential to increase ammonia levels than other diuretics.
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors or angiotensin-II antagonists:
enhanced hypotensive effect; risk of severe
hyperkalaemia.
Antibacterials: avoid with lymecycline.
Antidepressants: increased risk of postural
hypotension with tricyclics.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect with
post-synaptic alpha-blockers.
Cardiac glycosides: increased digoxin concentration.
Ciclosporin: increased risk of hyperkalaemia.
Cytotoxics: avoid with mitotane; increased risk
of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium: reduced lithium excretion.
NSAIDs: increased risk of hyperkalaemia (especially
with indometacin); increased risk of nephrotoxicity;
diuretic effect of spironolactone antagonised by
aspirin.
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia.
Metabolism
Spironolactone is rapidly and extensively metabolized to form different metabolites. A group of metabolites are formed when sulfur of spironolactone is removed, such as canrenone. Sulfur is retained in another group of metabolites, including 7-alpha (α)-thiomethylspironolactone (TMS) and 6-beta (?)-hydroxy-7-alpha (α)-thiomethylspirolactone (HTMS).
Spironolactone is firstly deacetylated to 7-α-thiospironolactone. 7-α-thiospironolactone is S-methylated to TMS, which is the primary metabolite, or dethioacetylated to canrenone. TMS and HTMS can be further metabolized.
In humans, the potencies of TMS and 7-α-thiospirolactone in reversing the effects of the synthetic mineralocorticoid, fludrocortisone, on urinary electrolyte composition were approximately a third relative to spironolactone. However, since the serum concentrations of these steroids were not determined, their incomplete absorption and/or first-pass metabolism could not be ruled out as a reason for their reduced in vivo activities.
Metabolism
Spironolactone is poorly absorbed after oral administration and has a delayed onset of action; it may take several days until a peak effect is produced. It has a somewhat slower onset of action than triamterene and amiloride (discussed later), but its natriuretic effect is modestly more pronounced, especially during long-term therapy. Spironolactone is rapidly and extensively metabolized, largely to the active metabolite canrenone. Canrenone and potassium canrenoate, its K+ salt, are available for clinical use in some countries outside the United States. Canrenone has a half-life of approximately 10 to 35 hours.The metabolites of spironolactone are excreted in both the urine and feces. New selective aldosterone receptor antagonists (SARA), such as eplerenone, have been developed but have not yet been introduced into clinical practice. Eplerenone and canrenone exhibit fewer steroidlike side effects (gynecomastia, hirsutism).
Storage
Room temperature
Structure and conformation
Synthetic steroid that resembles aldosterone.
Properties of Spironolactone
| Melting point: | 207-208 °C (lit.) |
| Boiling point: | 504.87°C (rough estimate) |
| alpha | -37 º (c=1, CHCl3) |
| Density | 1.1061 (rough estimate) |
| refractive index | -36 ° (C=1, CHCl3) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in ethanol (96 per cent). |
| appearance | white to light tan crystalline powder |
| form | Powder |
| color | White to yellow-white |
| Water Solubility | practically insoluble |
| Merck | 14,8760 |
| CAS DataBase Reference | 52-01-7(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 79) 2001 |
| NIST Chemistry Reference | Spironolactone(52-01-7) |
| EPA Substance Registry System | Spironolactone (52-01-7) |
Safety information for Spironolactone
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Spironolactone
Spironolactone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Spironolactone 99%View Details
Spironolactone 99%View Details -
 Spironolactone extrapure CAS 52-01-7View Details
Spironolactone extrapure CAS 52-01-7View Details
52-01-7 -
 Spironolactone 98% (HPLC) CAS 52-01-7View Details
Spironolactone 98% (HPLC) CAS 52-01-7View Details
52-01-7 -
 Spironolactone 99% (HPLC) CAS 52-01-7View Details
Spironolactone 99% (HPLC) CAS 52-01-7View Details
52-01-7 -
 Spironolactone CAS 52-01-7View Details
Spironolactone CAS 52-01-7View Details
52-01-7 -
 Spironolactone CAS 52-01-7View Details
Spironolactone CAS 52-01-7View Details
52-01-7 -
 Spironolactone CAS 52-01-7View Details
Spironolactone CAS 52-01-7View Details
52-01-7 -
 Spironolactone CAS 52-01-7View Details
Spironolactone CAS 52-01-7View Details
52-01-7
