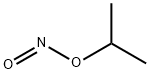
tert-Butyl nitrite
- CAS NO.:540-80-7
- Empirical Formula: C4H9NO2
- Molecular Weight: 103.12
- MDL number: MFCD00002055
- EINECS: 208-757-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
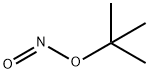
What is tert-Butyl nitrite?
Description
tert-Butyl nitrite is a common reagent used for diazotization, typically for Sandmeyer reactions. Isoamyl nitrite is used for similar purposes.
Chemical properties
clear yellow liquid
The Uses of tert-Butyl nitrite
tert-Butyl nitrite is employed as a reagent for diazotization and nitrosation of alcohols, thiols, amines and cycloalkanes. It plays an important role in photocatalyzed conversion of aryl and heteroarylamines to selenides. It finds application in the preparation of aryl azides from aryl amines. It is also useful for radical multifunctionalization reactions of aliphatic alkenes and source of nitric oxide.
The Uses of tert-Butyl nitrite

To ACN (350 mL) at RT was added CuBr2 (67 g, 300 mmol). The mixture was cooled to 0-5 C and was treated dropwise with t-BuONO (72.5 mL, 535 mmol). The mixture was stirred for 5 min, then was added the SM (35 g, 238 mmol). The reaction was stirred at RT for 2 h. EtOAc (525 mL) was added to the mixture and the reaction was quenched with 10% aq NH4Cl (350 mL) and 2% aq NH3 (175 mL). The layers were separated and the aq layer was extracted with EtOAc. The combined organics were dried and concentrated. The resulting material was purified by silica gel chromatography (eluting with 5% EtOAc/hexane) to provide the product as a yellow solid. [35 g, 70%]
The Uses of tert-Butyl nitrite
Jet propellant.
General Description
tert-Butyl nitrite (TBN) is an efficient NO source. TBN participates in photocatalyzed conversion of aryl- and heteroarylamines to selenides. It also participates in radical multifunctionalization reactions of aliphatic alkenes.
Purification Methods
If it is free from OH bands (IR) then distil it through a 12inch helices packed column under reduced pressure, otherwise wash with aqueous 5% NaHCO3 (effervescence), then H2O, dry (Na2SO4) and fractionate it through a 10 theoretical plates column at ca 10mm pressure. [Allen J Chem Soc 1968 1954, Coe & Doumani J Am Chem Soc 70 1516 1948, UV: Ungnade & Smiley J Org Chem 21 993 1956, IR: Terte Bull Soc Chim Belg 60 240 1951, Beilstein 1 IV 1622.]
Properties of tert-Butyl nitrite
| Boiling point: | 61-63 °C(lit.) |
| Density | 0.867 g/mL at 25 °C(lit.) |
| vapor pressure | 13.466kPa at 25℃ |
| refractive index | n |
| Flash point: | −10 °F |
| storage temp. | 2-8°C |
| solubility | alcohol: very soluble(lit.) |
| form | Liquid |
| color | Clear yellow |
| Water Solubility | slightly soluble |
| Merck | 14,1583 |
| BRN | 1209339 |
| CAS DataBase Reference | 540-80-7(CAS DataBase Reference) |
| NIST Chemistry Reference | (CH3)3CONO(540-80-7) |
Safety information for tert-Butyl nitrite
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for tert-Butyl nitrite
tert-Butyl nitrite manufacturer
NSR Amino Organics
Sainor Laboratories Pvt Ltd Unit III
SAKEM LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 t-Butylnitrite 540-80-7 98%View Details
t-Butylnitrite 540-80-7 98%View Details
540-80-7 -
 540-80-7 Tert-Butyl Nitrite 99%View Details
540-80-7 Tert-Butyl Nitrite 99%View Details
540-80-7 -
 tert-Butyl nitrite CAS 540-80-7View Details
tert-Butyl nitrite CAS 540-80-7View Details
540-80-7 -
 tert-Butyl Nitrite CAS 540-80-7View Details
tert-Butyl Nitrite CAS 540-80-7View Details
540-80-7 -
 tert-Butyl nitrite, 90% CAS 540-80-7View Details
tert-Butyl nitrite, 90% CAS 540-80-7View Details
540-80-7 -
 tert-butyl nitrite 80-85% CAS 540-80-7View Details
tert-butyl nitrite 80-85% CAS 540-80-7View Details
540-80-7 -
 tert-Butyl nitrite CAS 540-80-7View Details
tert-Butyl nitrite CAS 540-80-7View Details
540-80-7 -
 tert-Butyl nitrite CAS 540-80-7View Details
tert-Butyl nitrite CAS 540-80-7View Details
540-80-7
