Tanshinone IIA
Synonym(s):1,6,6-Trimethyl-6,7,8,9-tetrahydrophenanthro[1,2-b]furan-10,11-dione;Dan Shen ketone
- CAS NO.:568-72-9
- Empirical Formula: C19H18O3
- Molecular Weight: 294.34
- MDL number: MFCD00238692
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 16:50:29
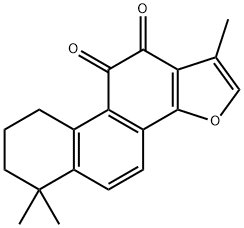
What is Tanshinone IIA?
Description
Tanshinone IIA mainly exists in the roots of Salvia miltiorrhiza Bge. Traditional Chinese medicine Danshen, slightly cold and bitter, is often used for activating blood circulation and eliminating stasis in Chinese medicine. It can also promote blood circulation, open the energy channels, calm the heart, cool the blood, relieve swelling, remove twinge in the heart and stomach, and remove carbuncle and erysipelas. It is classified as high grade in Shen Nong’s Herbal Classic. It has been used as a traditional Chinese medicine in clinical practice for thousands of years. And it is not only recorded in the leading medical works through the ages but also in CP and USP.
Description
Tanshinone IIa is one of the most abundant constituents of Chinese sage (Salvia miltiorrhiza) roots, which are highly prized in traditional medicine for their ability to treat cardiovascular disease and stroke. In additional to its traditional uses, tanshinone IIA is under study for anti-cancer treatments.
Yet another use for tanshinone IIA is being investigated. S. A. Renshaw and colleagues at the University of Sheffield found that it causes neutrophils to move away from animals'' injury sites, allowing the wounds to heal. Neutrophils are sent by the immune system to reduce inflammation, but if they stay around, healing can be delayed. Tanshinone IIA''s mechanism for removing neutrophils is under study.
Description
Tanshinone IIA (TSA) is a major lipophilic component of extracts from the root of S. miltiorrhiza, used widely in Chinese herbal medicine. It has anti-
Chemical properties
Orange powder
Physical properties
Appearance: brick-red crystalline powder. Melting point: 209–210?°C. Solubility: absorbs moisture easily, very soluble in hot water, slightly soluble in methanol or ethanol, and practically insoluble in chloroform
History
The study of the chemical composition of Salvia miltiorrhiza began in the 1930s. Japanese scholars first extracted three kinds of liposoluble components from Danshen. They are tanshinones I, II, and III.
The Uses of Tanshinone IIA
Tanshinone IIA is a derivative of Tanshinone and acts as a potential inhibitor of tumor growth in vivo. Also maintains vasodilating activity in vivo.
The Uses of Tanshinone IIA
antineoplastic, bone resorption inhibitor, antiproliferative, apoptosis inducer
What are the applications of Application
Tanshinone IIA is a transcription factor inhibitor
Definition
ChEBI: 1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g]benzofuran-10,11-dione is an abietane diterpenoid.
Synthesis Reference(s)
Journal of the American Chemical Society, 111, p. 1522, 1989 DOI: 10.1021/ja00186a070
Biochem/physiol Actions
Phenanthrenequinone constituent of Chinese medicinal herb Danshen (Salvia miltiorrhiza). Anti-inflammatory. Antioxidant. Cytotoxic against a variety of cell lines, inlcuding human glioma cells.
Pharmacology
Long-term researches show that tanshinone exhibits a variety of pharmacological effects, such as the protection of the cardiovascular system, anti-infective effects, and antioxidant effects. As for the domestic pharmacological study of tanshinone IIA, it was first started in the Shanghai Institute of Chinese Medicine. Professor Ding Guangsheng discussed the cardiovascular effects of tanshinone IIA.?He found that intraperitoneal injection of tanshinone IIA sodium sulfonate (200?mg/kg) significantly prolonged the survival time of mice under hypoxia atmosphere, and an increase in cardiac output was observed in a dog under anesthesia with a one-time intravenous injection of 20? mg/kg . In recent years, the mechanism of tanshinone IIA is increasingly brought to further research. Tanshinone IIA can increase the activity of superoxide dismutase (SOD) and interfere with the pathological process of many diseases, especially in cardiovascular diseases. It can attenuate the damages from the reactive oxygen species to vascular endothelial cells, lower the risk of atherosclerosis, and reduce the formation of atheromatous plaque. tumor cells and the expression of various genes connected with the proliferation, differentiation, and apoptosis of tumor cells. It may also have something to do with the inhibition of telomerase activity in tumor cells, changes in antigen expression on tumor cell surface, etc.
Clinical Use
Tanshinone IIA is a diterpenoid quinone liposoluble ingredient of high content, and its chemical structure is the most representative in Danshen. In addition to tanshinone IIA sodium sulfonate, there are a variety of preparations of Salvia common ketone used clinically, such as tanshinone tablets, tanshinone capsules, tanshinone injection, Danshen Shuxin capsule, compound Danshen soft capsules, and compound Danshen particles.
Cytotoxicity
IC50 (μg/mL): 0.59 (A549), 0.81 (TOV-21G) and 1.9 (MIAPaCa-2), NS (MV-3)(Chang et al. 2013; Fronza et al. 2011).
IC50 (μg/mL): 2.97 (HeLa), 2.71 (KB-3-1),>2.94 (NCI-H460), 2.62 (PC3), 2.94(MCF-7), 1.62 (K562)(Wu et al. 2014)
References
1) Kang et al. (2000), Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza; Immunopharmacology, 49 355 2) Sung et al. (1999), Tanshinone IIA, an ingredient of Salvia miltiorrhiza BUNGE, induces apoptosis in human leukemia cell lines through the activation of caspase-3; Exp. Mol. Med., 31 174 3) Park et al. (1999), Suppression of AP-1 Activity by Tanshinone and Cancer Cell Growth Inhibition; Bull. Korean Chem. Soc., 20 925
Properties of Tanshinone IIA
| Melting point: | 196.0 to 200.0 °C |
| Boiling point: | 480.7±44.0 °C(Predicted) |
| Density | 1.209±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | methanol: soluble5mg/mL, clear, red-orange to red |
| form | powder, red |
| color | Orange |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 568-72-9(CAS DataBase Reference) |
Safety information for Tanshinone IIA
Computed Descriptors for Tanshinone IIA
| InChIKey | HYXITZLLTYIPOF-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Tanshinone IIA CAS 568-72-9View Details
Tanshinone IIA CAS 568-72-9View Details
568-72-9 -
 Tanshinone iia 95% CAS 568-72-9View Details
Tanshinone iia 95% CAS 568-72-9View Details
568-72-9 -
 Tanshinone IIA 98% (HPLC) CAS 568-72-9View Details
Tanshinone IIA 98% (HPLC) CAS 568-72-9View Details
568-72-9 -
 Tanshinone IIA CAS 568-72-9View Details
Tanshinone IIA CAS 568-72-9View Details
568-72-9 -
 Tanshinone IIA CAS 568-72-9View Details
Tanshinone IIA CAS 568-72-9View Details
568-72-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1