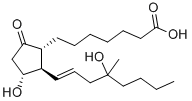
Sulprostone
Synonym(s):(5Z,11α,13E,15R)--11,15-Dihydroxy-9-oxo-16-phenoxy-17,18,19,20-tetranorprosta-5,13-dienoic acid methane sulfonamide;CP-34089;SHB-286;ZK-57671
- CAS NO.:60325-46-4
- Empirical Formula: C23H31NO7S
- Molecular Weight: 465.56
- MDL number: MFCD00216056
- EINECS: 262-173-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
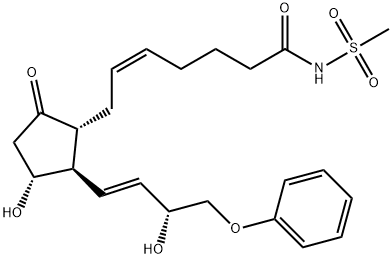
What is Sulprostone?
Description
Sulprostone is a metabolism resistant synthetic analog of PGE2. It is a selective agonist for EP3 receptors with a Ki value of 0.35 nM at the human recombinant EP3-
Originator
Nalador,Schering,W. Germany,1981
The Uses of Sulprostone
Sulprostone is a selective agonist of the PGE2 receptor.
The Uses of Sulprostone
antimalarial activity
The Uses of Sulprostone
Human chondrocytes3 and mouse adrenal chromaffin cells4 were treated with sulprostone to study the biological effects of PGE2.
What are the applications of Application
Sulprostone is a metabolism resistant synthetic analog of PGE2 that is a selective EP3 prostanoid receptor agonist
Definition
ChEBI: Sulprostone is a prostanoid.
Manufacturing Process
9α-Hydroxy-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-
13-ω-tetranorprostadienoicacid: To a solution of 1.6 g (3.6 mmols) (4-
carbohydroxy-n-butyl)triphenylphosphonium bromide in a dry nitrogen
atmosphere in 6.0 ml dry dimethyl sulfoxide was added 3.24 ml (6.5 mmols)
of a 2.0 M solution of sodium methylsulfinylmethide in dimethyl sulfoxide. To
this red ylide solution was added dropwise a solution of 613 mg (1.29 mmols)
2-[5α-hydroxy-3α-(tetrahydropyran-2-yloxy)-2α-(3α-tetrahydropyran-2-yloxy-
4-phenoxytrans-1-buten-1-yl)cyclopent-1α-yl] acetaldehyde, γ-hemiacetal in
5.0 ml dry dimethyl sulfoxide over a period of 20 minutes.
After an additional 2 hours stirring at room temperature, the reaction mixture
was poured onto ice water. The basic aqueous solution was washed twice with
ethyl acetate (20 ml) and acidified to pH 3 with 10% aqueous hydrochloric
acid.
The acidic solution was extracted with ethyl acetate (3 x 20 ml) and the
combined organic extracts washed once with water (10 ml), dried (MgSO4)
and evaporated to a solid residue. This solid residue was triturated with ethyl
acetate and the filtrate concentrated. Yield: 754 mg of 9α-hydroxy-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-
ωtetranorprostadienoic acid was collected.
9-Oxo-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-ω-
tetranorprostadienoicacid: To a solution cooled to -10°C under nitrogen of 754
mg (1.3 mmols) 9α-hydroxy-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-
phenoxy-cis-5-trans-13-ω-tetranorprostadienoic acid in 13 ml reagent grade
acetone was added dropwise to 0.56 ml (1.41 mmols) of Jones' reagent
(chromic anhydride). After 20 minutes at -10°C, 0.260 ml 2-propanol was
added and the reaction mixture was allowed to stir an additional 5 minutes at
which time it was combined with 75 ml ethyl acetate, washed with water (3 x
10 ml), dried (MgSO4)and concentrated to give 752 mg of 9-oxo-11α,15α-bis-
(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-ω-tetranorprostadienoic
acid, which was chromatographed on silica gel using ethyl acetate as eluent to
afford 505 mg of pure intermediate.
N-Methanesulfonyl-9-oxo-11α,15α-dihydroxy-5-cis-13-trans-16-phenoxy-ω-
tetranorprostadienamide:To 1.0 mmols of 9-oxo-11α,15α-bis-
(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-ω-tetranorprostadienoic
acid in 40 ml THF is added 2 ml triethylamine. After 15 minutes of stirring at
room temperature 10.0 ml of 0.1 M methanesulfonylisocyanate in THF is
added. After a further 1 hour of stirring, the reaction mixture is neutralized
with acetic acid and the solvent removed by evaporation (in vacuo). The
resultant residue is taken up in methylene chlorine and washed successively
with water and sodium bicarbonate to yield, after drying and solvent
evaporation, N-methanesulfonyl-9-oxo-11α,15α-bis-(tetrahydropyran-2-yloxy)-
16-phenoxy-cis-5-trans-13-ω-tetranorprostadienamide. This intermediate is
then hydrolyzed overnight with acetic acid/water and purified by column
chromatography to give the desired N-methanesulfonyl-9-oxo-11α,15α-
dihydroxy-5-cis-13-trans-16-phenoxy-ω-tetranorprostadienamide.
Therapeutic Function
Contraceptive
World Health Organization (WHO)
Sultopride, a neuroleptic indicated for the treatment of acute and chronic psychoses, was introduced on the market in 1976. In the early 1990s, its use was associated with cardiac arrhythmias, some of which were fatal. This led the regulatory authority in France to take restrictive action on the product. Sultopride continues to be marketed in several other countries.
Biochem/physiol Actions
Sulprostone is an analog of prostaglandin E2 (PGE2)1 and antagonizes vasopressin-induced antidiuretic responses in cells from rat renal inner medullae by a mechanism that involves activation of Rho.2
storage
Store at -20°C
Properties of Sulprostone
| Melting point: | 79.25°C |
| Density | 1.1918 (rough estimate) |
| refractive index | 1.5650 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: >5 mg/mL |
| form | solid |
| pka | 4.63±0.40(Predicted) |
| color | white to off-white |
Safety information for Sulprostone
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Sulprostone
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran


You may like
-
 Sulprostone CAS 60325-46-4View Details
Sulprostone CAS 60325-46-4View Details
60325-46-4 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0