SULFAPHENAZOLE
Synonym(s):SP;TFF2;Trefoil Factor 2;Trefoil Factor peptide;4-Amino-N-(1-phenyl-1H-pyrazol-5-yl)benzenesulfonamide
- CAS NO.:526-08-9
- Empirical Formula: C15H14N4O2S
- Molecular Weight: 314.36
- MDL number: MFCD00057226
- EINECS: 208-384-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
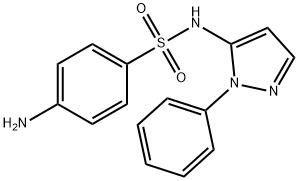
What is SULFAPHENAZOLE?
Description
CYP2C9 is a major cytochrome P450 enzyme that is involved in the metabolic clearance of various therapeutic agents. Disruption of this enzyme’s activity can lead to adverse drug reactions. Sulfaphenazole is an inhibitor of CYP2C9 (Ki = 0.3 μM) that demonstrates at least 100-
Chemical properties
White to off-white powder
The Uses of SULFAPHENAZOLE
Sulfaphenazole is used as an inhibitor for mammalian CYP2C9, an enzyme of the cytochrome P450 family allowing for pharmacological application. Sulfaphenazole can help protect against ischemia-reperfus ion, and resulting tissue damage by inhibition of CYP2C9.
The Uses of SULFAPHENAZOLE
Sulfaphenazole is used as an inhibitor for mammalian CYP2C9, an enzyme of the cytochrome P450 family allowing for pharmacological application.Sulfaphenazole can help protect against ischemia-reperfusion, and resulting tissue damage by inhibition of CYP2C9.
The Uses of SULFAPHENAZOLE
Sulfaphenazole has been used as a positive control to inhibit cytochrome P450 2C9 (cyp2c9) to quantify Rhodiola rosea inhibition. It has also been used as cytochrome P450 2C9 (cyp2c9) inhibitor in endothelial cells and microsomal preparations.
Background
Sulfaphenazole is a sulfonamide antibacterial.
Indications
For the treatment bacterial infections.
What are the applications of Application
Sulfaphenazole is a specific inhibitor of CYP2C9
Definition
ChEBI: A sulfonamide that is sulfanilamide in which the sulfonamide nitrogen is substituted by a 1-phenyl-1H-pyrazol-5-yl group. It is a selective inhibitor of cytochrome P450 (CYP) 2C9 isozyme, and antibacterial agent.
Biochem/physiol Actions
Antibacterial. Specific inhibitor of CYP2C9. Blocks pro-inflammatory and atherogenic effects of linoleic acid (increase in oxidative stress and activation of AP-1) mediated by CYP2C9. Specific inhibitor of CYP2C9. Blocks pro-inflammatory and atherogenic effects of linoleic acid (increase in oxidative stress and activation of AP-1) mediated by CYP2C9. Inhibits bradykinin-induced tPA release.
in vitro
in yeast expressed human cytochromes p450 of the 1a, 3a, and 2c subfamilies, sulfaphenazole acts as a strong and competitive inhibitor of cyp 2c9 with the ki value of 0.3 ± 0.1 μm. the ki values of sulfaphenazole for cyp 2c8 and 2c18 were 63 and 29 μm, respectively. sulfaphenazole failed to inhibit cyp 1a1, 1a2, 3a4, and 2c19 [1].
in vivo
in diabetic male mice (db/db strain), daily intraperitoneal injections of either the cyp 2c inhibitor sulfaphenazole (5.13 mg/kg) for 8 weeks, sulfaphenazole restored endothelium-mediated relaxation in db/db mice. sulfaphenazole reduced oxidative stress, increased no bioavailability and restored endothelial function in db/db mice [3].
Metabolism
Hepatic.
Purification Methods
Crystallise it from EtOH or aqueous EtOH. [Schmidt & Druey Helv Chim Acta 41 309 1958, Beilstein 25 III/IV 2029.]
References
[1] mancy a, dijols s, poli s, et al. interaction of sulfaphenazole derivatives with human liver cytochromes p450 2c: molecular origin of the specific inhibitory effects of sulfaphenazole on cyp 2c9 and consequences for the substrate binding site topology of cyp 2c9[j]. biochemistry, 1996, 35(50): 16205-16212.
[2] rettie a e, jones j p. clinical and toxicological relevance of cyp2c9: drug-drug interactions and pharmacogenetics[j]. annu. rev. pharmacol. toxicol., 2005, 45: 477-494.
[3] elmi s, sallam n a, rahman m m, et al. sulfaphenazole treatment restores endothelium-dependent vasodilation in diabetic mice[j]. vascular pharmacology, 2008, 48(1): 1-8.
Properties of SULFAPHENAZOLE
| Melting point: | 179-183 °C |
| Boiling point: | 541.9±56.0 °C(Predicted) |
| Density | 1.39 |
| refractive index | 1.6440 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 5.71(H2O
t = 25
I = 0.05) (Uncertain) |
| color | white to light yellow |
Safety information for SULFAPHENAZOLE
Computed Descriptors for SULFAPHENAZOLE
SULFAPHENAZOLE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Sulfaphenazole 95% CAS 526-08-9View Details
Sulfaphenazole 95% CAS 526-08-9View Details
526-08-9 -
 Sulfaphenazole CAS 526-08-9View Details
Sulfaphenazole CAS 526-08-9View Details
526-08-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8