Strontium sulfate
Synonym(s):Celestite
- CAS NO.:7759-02-6
- Empirical Formula: O4SSr
- Molecular Weight: 183.68
- MDL number: MFCD00036274
- EINECS: 231-850-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-26 17:40:57
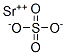
What is Strontium sulfate?
Description
Strontium sulfate has the molecular formula of SrSO4
and the molecular weight of 183.6842 g/mol. It can be
prepared by the reaction of sodium sulfate and strontium
chloride:
Na2SO4+SrCl2?SrSO4+2NaCl
Strontium sulfate occurs in nature as “celestite” and is
mined from its natural deposits. Also, the sulfate can be
made by reacting strontium oxide, hydroxide, or
carbonate with dilute hydrochloric acid:
SrO+H2SO4?SrSO4+H2O
SrCO3+H2SO4?SrSO4+CO2+H2O
No hydrates are known.Its hardness is 3.3
Mohs. It melts at 1608°C. Its solubility in water is
0.015 g/100 ml at 25°C and 0.014 g at 35°C. It is insoluble
in ethanol and acetone, but slightly soluble in acids
and alkalis. It is soluble in alkali chloride solutions. The heat of formation, △Hf, is –1453 kJ/mol with an entropy
value, S0, of 117.0 J/mol K. Its Mohs Hardness is 3.0–3.5.
A water solution has a pH of 7.5. Its specific gravity ranges from 3.8 to 4.0 g/cm3.Pure strontium sulfate precipitate consists of single crystals, about
some tens of microns long, with distinctly visible growth
steps. Crystals grown with polyvinyl sulfonate in stirred
solutions show a striking change of habit: they are
sponge-like structures, composed of minute crystallites,
of size in the nanometer range.
Crystallized strontium sulfate is utilized by a small
group of protozoa as a main constituent of their skeleton.
It is sometimes used to impart iridescence to glass
and pottery glazes or used as a fining agent in crystal
glass.
There is very little demand for this salt in industry. It
is available in limited quantities commercially.
Chemical properties
Milky white powder
The Uses of Strontium sulfate
Widely applied in the manufacturing of Barium Salts, pigments, special glass, printing inks, paints, filler for textiles, linoleum, paper, rubber, base for color lakes, as pigment and white lead substitutes.
The Uses of Strontium sulfate
In ceramics, pyrotechnics.
The Uses of Strontium sulfate
Strontium Sulfate combined with Bi-2212 creates a superconductor.
Definition
strontium sulphate: A white solid,SrSO4; r.d. 3.96; m.p. 1605°C. It canbe made by dissolving strontiumoxide, hydroxide, or carbonate in sulphuricacid. It is used as a pigment inpaints and ceramic glazes and to providea red colour in fireworks.
Flammability and Explosibility
Non flammable
Properties of Strontium sulfate
| Melting point: | 1606 °C (lit.) |
| Density | 3.96 g/mL at 25 °C (lit.) |
| RTECS | WT1210000 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | aqueous acid: slightly soluble(lit.) |
| form | powder |
| color | White |
| Water Solubility | Soluble in dilute hydrochloric acid and nitric acid . Slightly soluble in water < 0.01 g/L . Insoluble in ethanol and alkalis. |
| Merck | 14,8848 |
| Solubility Product Constant (Ksp) | pKsp: 6.46 |
| CAS DataBase Reference | 7759-02-6(CAS DataBase Reference) |
| EPA Substance Registry System | Sulfuric acid, strontium salt (1:1) (7759-02-6) |
Safety information for Strontium sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Strontium sulfate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 7759-02-6 98%View Details
7759-02-6 98%View Details
7759-02-6 -
 Strontium sulfate, For reagent grade CAS 7759-02-6View Details
Strontium sulfate, For reagent grade CAS 7759-02-6View Details
7759-02-6 -
 Strontium sulfate, For reagent grade CAS 7759-02-6View Details
Strontium sulfate, For reagent grade CAS 7759-02-6View Details
7759-02-6 -
 Strontium sulfate CAS 7759-02-6View Details
Strontium sulfate CAS 7759-02-6View Details
7759-02-6 -
 Strontium sulfate CAS 7759-02-6View Details
Strontium sulfate CAS 7759-02-6View Details
7759-02-6 -
 STRONTIUM SULPHATE 99%View Details
STRONTIUM SULPHATE 99%View Details -
 Strontium sulphate CAS 7759-02-6View Details
Strontium sulphate CAS 7759-02-6View Details
7759-02-6 -
 STRONTIUM SULPHATE ANHYDROUS Extra Pure CASView Details
STRONTIUM SULPHATE ANHYDROUS Extra Pure CASView Details
