Strontium ranelate
Synonym(s):2-[N,N-Di(carboxymethyl)amino]-3-cyano-4-carboxymethylthiophene-5-carboxylic acid strontium salt;5-[Bis(carboxymethyl)amino]-2-carboxy-4-cyano-3-thiopheneacetic acid, strontium salt;Distrontium renelate;Ranelic acid strontium salt
- CAS NO.:135459-87-9
- Empirical Formula: C12H12N2O8SSr
- Molecular Weight: 431.91
- MDL number: MFCD09038742
- EINECS: 690-882-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
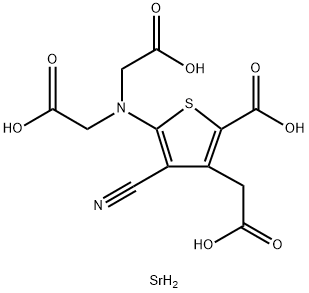
What is Strontium ranelate?
Absorption
The absolute bioavailability of strontium is about 25% (within a range of 19-27%) after an oral dose of 2 g strontium ranelate. Maximum plasma concentrations are reached approximately 3-5 hours after a single dose of 2 g. Steady state is reached after 2 weeks of treatement. The intake of strontium ranelate with calcium or food reduces the bioavailablity of strontium ranelate by about 60-70%, compared with administration 3 hours after a meal .
Due to the relatively slow absorption of strontium, food and calcium intake should be avoided both before and after administration of strontium ranelate. Conversely, oral supplementation with vitamin D has no effect on strontium exposure whatsoever.
Toxicity
Strontium ranelate has been withdrawn worldwide owing to an increased adverse cardiac effects profile along with increased risk of venous thromboembolism (VTE) and various life threatening allergic reactions.
In pooled randomised placebo-controlled studies of post-menopausal osteoporotic patients, a significant increase in myocardial infarction has been observed in patients treated with strontium ranelate compared to placebo . Patients with significant risk factors for cardiovascular events (ie. hypertension, hyperlipidemia, diabetes mellitus, smoking) would be susceptible to an even higher risk of cardiac ishaemic events like myocardial infarction .
In phase III placebo-controlled studies, strontium ranelate treatment was associated with an increase in the annual incidence of venous thromboembolism (VTE), including pulmonary embolism. This places substantial risk on patients at risk of VTE and elderly (over 80 years) patients at risk of VTE who may be more commonly associated with illnesses or conditions leading to immobilisation .
Life-threatening cutaneous reactions like Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) have been reported with the use of strontium ranelate. In particular, a higher incidence of such reactions has been reported in patients of Asian origin.
In a pooled analysis of randomised placebo-controlled studies in post-menopausal osteoporotic patients, the most common adverse reactions consisted of nausea and diarrhea .
Nevertheless, good tolerance was shown in a clinical study investigating the repeated administration of 4 g strontium ranelate per day over 25 days in healthy postmenopausal women . Single administration of doses up to 11 g in healthy young male volunteers did not cause any particular symptoms .
In patients with mild to moderate renal impairment (30-70 ml/min creatine clearance), strontium clearance decreases as creatinine clearance decreases (approximately 30% decrease over the creatinine clearance range 30 to 70 ml/min) and thereby induces an increase in strontium plasma levels. However, no dosage adjustment is required for patients with miod to moderate renal impairment - although no pharmacokinetic data exists for patients with severe renal impairment associated with creatinine clearance below 30 ml/min .
There are no data from the use of strontium ranelate in pregnant women .
Physico-chemical data suggests strontium ranelate can be excreted into human milk. Strontium ranelate should not be used during breastfeeding .
No effects were observed on male and female fertility in animal studies .
Description
Strontium ranelate, a divalent strontium salt of ranelic acid, has been developed and launched for the treatment of osteoporosis. As early as 1910, investigations suggested that strontium stimulates the formation of osteoid tissues while simultaneously repressing the resorptive process in bones. Specifically, strontium enhances pre-osteoblastic cell replication, inhibits pre-osteoclast differentiation, and suppresses the bone-resorbng activity of osteoclasts. From the evaluation of 26 strontium salts, ranelic acid was selected as the ideal strontium carrier due to its physicochemical and pharmacokinetic properties. The thiophene core of ranelic acid is constructed by the condensation of dialkyl 3-oxoglutarate, malononitrile, and sulfur in a suitable alcohol in the presence of morpholine or diethylamine. The resultant diester of 5-amino-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid is subsequently dialkylated with an alkyl bromoacetate to provide the tetraester precursor to strontium ranelate. Strontium ranelate is supplied in a 2 g sachet, and the drug is evenly suspended in water prior to consumption. Since the simultaneous ingestion of either calcium or food has a negative influence on the bioavailability of strontium ranelate, it is recommended that strontium ranelate be administered once a day at bedtime. Following this regimen, the absolute bioavailability of strontium is 27% while that of ranelic acid is 2.5%. Because strontium ranelate dissociates after intake, and ranelic acid has negligible absorption, the effects of the drug on bone metabolism are dependent on the pharmacokinetics of strontium. In postmenopausal women, the half-life of strontium is 6.3±2.3 days, and renal clearance accounts for 57%of the total clearance of 12mL/min. After a 25-day treatment, the maximum plasma concentration of strontium is 20±2.3 mg/L. In addition, not only is perfect stability of strontium plasma concentration achieved within 3 to 24 months of chronic administration so is stabilization of strontium incorporation into bones. Strontium is incorporated into bone by two mechanisms. The predominant mode involves the rapid, saturable surface exchange with calcium. A slower mechanism embodies the incorporation of strontium into the crystal lattice of the bone mineral; however, only a small amount of calcium in the apatite is substituted by strontium at pharmacological doses. A phase II clinical trial assessed the effect of various strontium ranelate doses in postmenopausal women with established osteoporosis. The primary efficacy endpoint for this double-blind, randomized, placebo- controlled trial was the measure of mean lumbar bone mineral density (BMD) by dual-energy X-ray absorptiometry. A statistically significant dose-dependent increase in lumbar BMD was observed; increases of 1.3, 5.9, 8.3, and 13.6% were recorded for placebo, 500-, 1000-, and 2000-mg doses of strontium ranelate, respectively. In a phase III trial encompassing 1,649 osteoporotic postmenopausal women from 12 countries, the efficacy of a 2 g/day dose in preventing new vertebral fractures was evaluated. The mean lumbar BMD was 0.73 g/cm2 while the mean age at baseline was 70 years. All of the enrolled patients had at least one prior vertebral fracture. The primary end point for this study was a reduction in the incidence of patients experiencing fractures. While 222 women in the placebo group experienced a new vertebral fracture, only 139 patients treated with strontium ranelate presented with new fractures. Furthermore, the risk of fracture was reduced by 51% in the third year alone, implicating the sustained efficacy of the drug. For both the phase II and phase III studies, strontium ranelate was well tolerated with most of the adverse events being mild-to-moderate in severity. The most commonly reported events in all treatment groups were musculoskeletal disorders (back pain, arthralgia, and lumbar pain). As for laboratory measurements, only creatine phosphokinase, the musculoskeletal isoenzyme, was significantly elevated in the 1000-mg and 2000-mg strontium ranelate groups; however, this did not translate into any particular clinical or biological abnormality. Without relevant data regarding bone safety in patients with renal impairment, strontium ranelate is currently contraindicated in patients with creatine clearance below 30mL/min.
Chemical properties
Crystalline Solid
Originator
Fujisawa (Japan)
The Uses of Strontium ranelate
Bone metabolism modulator; inhibits bone resorption while maintaining bone formation. Antiosteoporotic.
The Uses of Strontium ranelate
Strontium ranelate (Protelos) is a strontium(II) salt of ranelic acid for (-)-desmethoxyverapamil binding to calcium channel with IC50 of 0.5 mM.
Indications
Strontium ranelate is therapeutically indicated for the treatment of severe osteoperosis in: a) postmenopasual women, and b) adult men, who are at high risk of fractures, for whom treatment with other medical products approved for the treatment of osteoperosis is not possible due to, for example, contraindications or intolerance.
In postmenopausal women, strontium ranelate can also reduce the risk of vertebral and hip fractures .
What are the applications of Application
Strontium Ranelate is a bone metabolism modulator
Background
Strontium ranelate, a strontium (II) salt of ranelic acid, is a medication for osteoporosis. Some reports have shown that strontium ranelate can slow down the progression of osteoarthritis of the knee. This agent presents an atypical mechanism of action in which it increases deposition of new bone by osteoblasts and, simultaneously, reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA) indicated for use in treatment of severe osteoporosis.
Furthermore, various clinical studies demonstrate the ability of strontium ranelate to improve and strengthen intrinsic bone tissue quality and microarchitecture in osteoporosis by way of a number of cellular and microstructural changes by which anti-fracture efficacy is enhanced.
Available for prescription use for a time in some parts of the world as Protelos (strontium ranelate) 2 g granules for oral suspension by Servier, it was ultimately discontinued in 2016-2017 owing to an increased adverse cardiac effects profile along with increased risk of venous thromboembolism (VTE) and various life threatening allergic reactions.
brand name
Protelos
Pharmacokinetics
In general, it is believed that strontium ranelate is capable of affecting a rebalance in bone turnover in favour of bone formation by: (1) increasing osteoblast differentiation from progenitors, osteoblast activity and survival, as well as regulating osteoblast-induced osteoclastogenesis, and (2) decreasing osteoclast differentiation and activity, as well as increasing osteoclast apoptosis .
It has also been shown that strontium ranelate is capable of improving and strengthening various components of overall bone tissue quality like bone mineral density and bone microarchitecture .
Clinical Use
Treatment of post menopausal osteoporosis and men at high risk of fractures
Drug interactions
Potentially hazardous interactions with other drugs
Calcium-containing compounds: separate
administration by at least 2 hours.
Antacids: separate administration by at least 2 hours.
Antibiotics: strontium can reduce absorption of oral
tetracycline and quinolones - suspend strontium
therapy during treatment.
Metabolism
Strontium ranelate has a high affinity for bone tissue. It is not metabolised, and excretion occurs via the kidneys and gastrointestinal tract.
Metabolism
As a divalent cation, strontium is not metabolized .
Properties of Strontium ranelate
| Melting point: | >310°C (dec.) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: soluble1mg/mL, clear (warmed) |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 135459-87-9 |
Safety information for Strontium ranelate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Strontium ranelate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 135459-87-9 Strontium ranelate 98%View Details
135459-87-9 Strontium ranelate 98%View Details
135459-87-9 -
 135459-87-9 98%View Details
135459-87-9 98%View Details
135459-87-9 -
 Strontium ranelate 98%View Details
Strontium ranelate 98%View Details
135459-87-9 -
 Strontium ranelate 135459-87-9 98%View Details
Strontium ranelate 135459-87-9 98%View Details
135459-87-9 -
 Strontium ranelate 95% CAS 135459-87-9View Details
Strontium ranelate 95% CAS 135459-87-9View Details
135459-87-9 -
 Strontium ranelate CAS 135459-87-9View Details
Strontium ranelate CAS 135459-87-9View Details
135459-87-9 -
 STRONTIUM RANELATEView Details
STRONTIUM RANELATEView Details
135459-87-9 -
 Strontium Ranelate C12H6N2O8SSr2View Details
Strontium Ranelate C12H6N2O8SSr2View Details
135459-87-9
