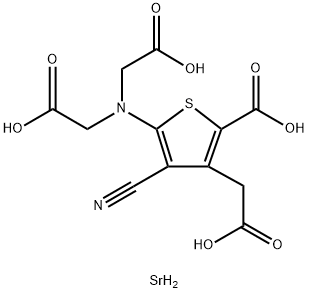
135459-87-9
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 513.5 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 513.7957108 g/mol |
| Monoisotopic Mass | 513.7957108 g/mol |
| Topological Polar Surface Area | 216 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 533 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Strontium ranelate, a strontium (II) salt of ranelic acid, is a medication for osteoporosis. Some reports have shown that strontium ranelate can slow down the progression of osteoarthritis of the knee. This agent presents an atypical mechanism of action in which it increases deposition of new bone by osteoblasts and, simultaneously, reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA) indicated for use in treatment of severe osteoporosis. Furthermore, various clinical studies demonstrate the ability of strontium ranelate to improve and strengthen intrinsic bone tissue quality and microarchitecture in osteoporosis by way of a number of cellular and microstructural changes by which anti-fracture efficacy is enhanced. Available for prescription use for a time in some parts of the world as Protelos (strontium ranelate) 2 g granules for oral suspension by Servier, it was ultimately discontinued in 2016-2017 owing to an increased adverse cardiac effects profile along with increased risk of venous thromboembolism (VTE) and various life threatening allergic reactions.
