Spermine
Synonym(s):N,N′-Bis(3-aminopropyl)-1,4-diaminobutane;Gerontine;Musculamine;Neuridine
- CAS NO.:71-44-3
- Empirical Formula: C10H26N4
- Molecular Weight: 202.34
- MDL number: MFCD00012914
- EINECS: 200-754-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
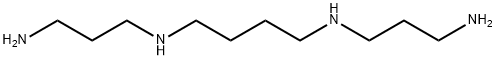
What is Spermine?
Description
Spermine is an endogenous polyamine synthesized from the reaction of spermidine with decarboxylated S-adenosylmethionine in the presence of the enzyme spermine synthase and is required for eukaryotic cell growth and protein synthesis. Intracellular spermine blocks inward rectifying K+ channels, whereas extracellular spermine acts as a mixed NMDA glutamate receptor agonist/antagonist at the polyamine site. Spermine has neuroprotective and anti-inflammatory effects and functions as a free radical scavenger to prevent DNA damage by reactive oxygen species.
Description
The tetraamine spermine is biosynthesized from the triamine spermidine, which is produced from the diamine putrescine. Anton van Leeuwenhoek, the microscopy pioneer, described spermine crystals in human semen in 1678, and spermidine was also first detected in sperm. Despite their names, both polyamines are ubiquitous in organisms and have several biological functions.
Chemical properties
White to slightly off-white powder crysta
The Uses of Spermine
Binds to the polyamine modulatory site of NMDA Spermine is essential for both normal and neoplastic tissue growth. It is involved in the modulation of calcium-dependent immune processes. It plays an important role in cellular proliferation and differentiation as well as inhibits neuronal nitric oxide synthase (nNOS).
The Uses of Spermine
Biogenic polyamine formed from spermidine and occurring in almost all tissues. Essential for both normal and neoplastic tissue growth. Involved in the modulation of calcium-dependent immune processes
The Uses of Spermine
immune modulator
What are the applications of Application
Spermine is a NOS1 inhibitor, NMDA activator, and GluR inhibitor
Definition
ChEBI: A polyazaalkane that is tetradecane in which the carbons at positions 1, 5, 10 and 14 are replaced by nitrogens. Spermine has broad actions on cellular metabolism.
General Description
Spermine?is a polyamine, which functions as a free radical scavenger. It modulates gene expression, chromatin stabilization and prevents DNA damage. Spermine inhibits endonuclease-mediated DNA fragmentation.
Biochem/physiol Actions
Mixed NMDA glutamate receptor agonist/antagonist at the polyamine site. Neuroprotective effects have been observed at high concentrations (1 mM), while neurotoxicity is observed at lower concentrations. It enhances agonist effectiveness at the strychnine-insensitive glycine site. Plays a role in cellular proliferation and differentiation; inhibits neuronal nitric oxide synthase (nNOS).
storage
+4°C (desiccate)
References
[1] fakler b, brndle u, glowatzki e, et al. strong voltage-dependent inward rectification of inward rectifier k+ channels is caused by intracellular spermine[j]. cell, 1995, 80(1): 149-154.
[2] til h p, falke h e, prinsen m k, et al. acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats[j]. food and chemical toxicology, 1997, 35(3): 337-348.
Properties of Spermine
| Melting point: | 28-30 (lit.) |
| Boiling point: | 150 °C/5 mmHg (lit.) |
| Density | 0.937 |
| refractive index | 1.4850 (estimate) |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | powder |
| pka | 10.88±0.19(Predicted) |
| color | Colorless to off-white |
| Water Solubility | Soluble in chloroform, methanol, water and lower alcohols. Insoluble in ether, benzene and petroleum ether. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,8743 |
| BRN | 1751791 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 71-44-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Spermine(71-44-3) |
| EPA Substance Registry System | 1,4-Butanediamine, N,N'-bis(3-aminopropyl)- (71-44-3) |
Safety information for Spermine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Spermine
| InChIKey | PFNFFQXMRSDOHW-UHFFFAOYSA-N |
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Spermine CAS 71-44-3View Details
Spermine CAS 71-44-3View Details
71-44-3 -
 Spermine Free Base extrapure CAS 71-44-3View Details
Spermine Free Base extrapure CAS 71-44-3View Details
71-44-3 -
 Spermine, ≥90% CAS 71-44-3View Details
Spermine, ≥90% CAS 71-44-3View Details
71-44-3 -
 Spermine, ≥97% CAS 71-44-3View Details
Spermine, ≥97% CAS 71-44-3View Details
71-44-3 -
 Spermine CAS 71-44-3View Details
Spermine CAS 71-44-3View Details
71-44-3 -
 Spermine CAS 71-44-3View Details
Spermine CAS 71-44-3View Details
71-44-3 -
 Spermine CAS 71-44-3View Details
Spermine CAS 71-44-3View Details
71-44-3 -
 Spermine CAS 71-44-3View Details
Spermine CAS 71-44-3View Details
71-44-3