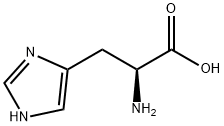
Histamine
Synonym(s):2-(4-Imidazolyl)ethylamine;Histamine, Free Base - CAS 51-45-6 - Calbiochem
- CAS NO.:51-45-6
- Empirical Formula: C5H9N3
- Molecular Weight: 111.15
- MDL number: MFCD00005210
- EINECS: 200-100-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
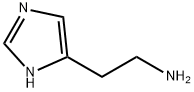
What is Histamine?
Absorption
Readily absorbed after parenteral administration.
Toxicity
LD50=807 mg/kg (mouse, oral). Side effects can lead to hypertension, hypotension, headache, dizziness, nervousness and tachycardia. Large overdoses can lead to seizures.
Description
Histamine is the chemical messenger that antihistamine medicines try to block. Histamine is released by mast cells during an allergy attack and can produce multiple biological effects including bronchoconstriction (asthma), vasodilation (watery eyes, runny nose), and gastric acid secretion.
Description
Histamine is an organic triamine that is a strong vasodilator found in blood and most bodily tissues. It is involved in inflammatory and immune responses. Histamine is stored primarily in mast cells and basophils; it is released in response to tissue damage caused by injury, infection, or allergens.
In 1938, French microbiologists Lévy-Brühl and Ungar showed that pneumococcus bacteria and Balantidium coli biosynthesize histamine from the amino acid histidine. It was later shown that this reaction is catalyzed by the enzyme histidine decarboxylase.
Histamine has many physiological functions, but this time of year we focus on its role in reactions to allergens such as pollen. Allergens bind to the antibody immunoglobulin E in the mucous membranes of the nasal cavity, releasing histamine, and leading to runny noses, watery eyes, sneezing, and nasal congestion. Fortunately, many antihistamines are available to combat these symptoms.
Chemical properties
White to slightly yellow powder
History
Histamine is an important protein involved in many allergic reactions. Allergies are caused by an immune response to a normally innocuous substance (i.e. pollen, dust) that comes in contact with lymphocytes specific for that substance, or antigen. The history of histamine and the development of antihistamines have been reviewed in [Drugs of Today (1986) and the Journal of Allergy & Clinical Immunology]. Histamine was the first to be characterized of a series of biogenic amines that are released in the inflammatory process. As early as 1910, it was shown that histamine caused constriction of isolated guinea pig ileum and, subsequently, it was found that histamine induced a shock-like syndrome. In 1927 the presence of histamine in normal tissues was demonstrated. Attempts to reduce histamine manifestations led to the report, in 1933, that certain phenolic ethers inhibited histamine action. Toxicity precluded clinical use. In 1942 phenbenzamine (Antergan), C17H22N2, was the first antihistamine to be successfully used in humans.
In 1966, the name H1 was proposed for receptors blocked by the at that time known antihistamines. It was also speculated that the other actions of histamine were likely to be mediated by other histamine receptors. The existence of the H2 receptor was accepted in 1972 and the H3 receptor was recognized in rat brain in 1983. H3 receptors in the brain appear to be involved in the feedback control of both histamine synthesis and release, whereas release of various other neurotransmitters, eg, serotinin (5-HT), dopamine, noradrenaline, and acetylcholine, is also modulated. H3 receptor effects have also been demonstrated in various peripheral tissues and H3 agonists and antagonists are undergoing intensive study for therapeutic applications.
The Uses of Histamine
Histamine inhibits the synthesis of IL-2 and γ-IFN in peripheral blood mononuclear cells and lipopolysaccharide-induced synthesis of TNF-α in monocytes via H2?receptor activation. It is a powerful stimulant of gastric secretion, a constrictor of bronchial smooth muscle, a vasodilator, and also a centrally acting neurotransmitter.
The Uses of Histamine
H1&2 agonist, edema induction, gastric secretion stimulant
What are the applications of Application
Histamine, free base is a compound that activates NOS which results in the release of NO
Indications
Histamine phosphate is indicated as a diagnostic aid for the evaluation of gastric acid secretory function.
Background
A depressor amine derived by enzymatic decarboxylation of histidine. It is a powerful stimulant of gastric secretion, a constrictor of bronchial smooth muscle, a vasodilator, and also a centrally acting neurotransmitter.
Definition
ChEBI: A member of the class of imidazoles that is 1H-imidazole substituted at position C-4 by a 2-aminoethyl group.
Indications
Sinus problems, hay fever, bronchial asthma, hives, eczema, contact dermatitis, food allergies, and reactions to drugs are all allergic reactions associated with the release of histamine and other autocoids, such as serotonin, leukotrienes, and prostaglandins. Histamine release is frequently associated with various inflammatory states and may be increased in urticarial reactions, mastocytosis, and basophilia. Histamine also acts as a neurotransmitter in the central nervous system (CNS). Upon release from its storage sites, histamine exerts effects ranging from mild irritation and itching to anaphylactic shock and eventual death.
Biosynthesis
Virtually all of the histamine found in individual organs
and tissues is synthesized locally and stored in subcellular
secretory granules. Within the tissues, the mast cells
are the principal sites of storage; in the blood, the basophils serve this function. Histamine is also present in
neurons of the CNS, where it acts as a neurotransmitter.
Histamine is synthesized from the amino acid histidine
by an action of the enzyme histidine decarboxylase. Following synthesis, histamine is either rapidly
inactivated or stored in the secretory granules of
mast cells and basophils as an inactive complex with
proteases and heparin sulfate or chondroitin sulfate.
Biological Functions
Histamine occurs in the brain, particularly in certain hypothalamic neurons, and evidence is strong that histamine is a neurotransmitter. Distribution of histamine, its synthetic enzyme (histidine decarboxylase), and methyl histamine (the major brain metabolite) is not uniform. Possible roles for histamine in the regulation of food and water intake, thermoregulation, hormone release, and sleep have been suggested.
General Description
Histamine is a neurotransmitter produced by neurons of the posterior hypothalamus. In the brain, histamine is predominantly present in the gray matter.
Biochem/physiol Actions
Histamine participates in innate and acquired immune response, mediating allergy and inflammation. It helps in intestinal muscle contraction. During anaphylactic shock histamine causes bronchial constriction. Histamine is also involved in gastric acid secretion, epithelial and endothelial barrier control.
Mechanism of action
Non–Antigen-Mediated Release of Histamine Histamine may be released from mast cells by mechanisms that do not require prior sensitization of the immune system. Drugs, high-molecular-weight proteins, venoms, and other substances that damage or disrupt cell membranes can induce the release of histamine. Any thermal or mechanical stress of sufficient intensity also will result in histamine release. Cytotoxic compounds, may release histamine as the result of disruption of cell membranes.
Pharmacokinetics
Histamine stimulates gastric gland secretion, causing an increased secretion of gastric juice of high acidity. This action is probably due mainly to a direct action on parietal and chief gland cells.
Pharmacology
Histamine is found in animal tissues and venoms and in many bacteria and plants.Within the human body, the largest histamine concentrations are in the skin, lungs, and gastrointestinal mucosa, while concentrations are smaller in almost all other organs and tissues.Histamine is present in human plasma at relatively low concentrations (usually less than 0.5 ng/mL); in contrast, wholeblood levels can be as high as 30-fold greater. Substantial quantities of histamine are present in urine, with excretion rates varying from 10 to 40μg per 24 hours.
Clinical Use
Histamine has only minor uses in clinical medicine. In the past it was used to diagnose pernicious anemia, in which histamine fails to evoke the usual secretion of gastric acid. Histamine has been used to assess bronchial hyperreactivity, although this test may be quite hazardous for asthmatics. Today the main clinical use of histamine is as a positive control injection for allergy skin testing.
Side Effects
Sedation is the most frequent adverse reaction to the
first-generation antihistamines. An additive effect on
alertness and motor skills will result if alcohol or another
depressant is taken with these drugs. Antimuscarinic
effects caused by these drugs include dry
mouth and respiratory passages, urinary retention, and
dysuria. Nausea, vomiting, constipation or diarrhea,
dizziness, insomnia, nervousness, and fatigue also have
been reported. Drug allergy, especially after topical application,
is fairly common.Tolerance to certain antihistamines
may develop after prolonged administration.
Teratogenic effects of the piperazine antihistamines
have been shown in animal studies. Epidemiological studies have not shown such an association in humans.
The effects of toxic doses of first-generation antihistamines,
similar to those seen following atropine administration,
include excitement, hallucinations, dry mouth,
dilated pupils, flushing, convulsions, urinary retention,
sinus tachycardia, coma, and death.
The second-generation H1-antagonists are often referred
to as nonsedating antihistamines; however, doses
above the usual therapeutic level can cause sleepiness
in certain individuals.A more serious adverse effect of
some earlier second-generation antihistamines is cardiotoxicity.
Synthesis
Histamine is synthesized in tissues by decarboxylation of amino acid L-histidine, a process catalyzed by the pyridoxalphosphate-dependent enzyme L-histidinedecarboxylase. Histamine can enter the organism with food; it also can be generated by bacteria of the gastrointestinal tract.
Metabolism
Primarily hepatic. Histamine is rapidly metabolized by methylation and oxidation. Methylation involves ring methylation and catalyzation by the enzyme histamine-N-methyltransferase, producing N-methylhistamine, which is mostly converted to N-methyl imidazole acetic acid. 2 to 3% excreted as free histamine, 4 to 8% as N-methylhistamine, 42 to 47% as N-methyl imidazole acetic acid, 9 to 11% as imidazole acetic acid, and 16 to 23% as imidazole acetic acid riboside.
Purification Methods
It crystallises from *benzene or chloroform. [Beilstein 25 I 628, 25 II 302, 25 III/IV 2049.]
Properties of Histamine
| Melting point: | 83-84 °C (lit.) |
| Boiling point: | 167 °C/0.8 mmHg (lit.) |
| Density | 0.9902 (rough estimate) |
| refractive index | 1.4690 (estimate) |
| storage temp. | -20°C |
| solubility | 34 g/l |
| solubility | DMSO: 20 mg/ml; Ethanol: 10 mg/ml; PBS (pH 7.2): 10 mg/ml |
| pka | 6.04(at 25℃) |
| appearance | white crystals or powder |
| form | Solid |
| color | White to light yellow |
| Water Solubility | Soluble in water, alcohol, and hot chloroform. |
| Merck | 13,4739 |
| BRN | 2012 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 51-45-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Histamine(51-45-6) |
| EPA Substance Registry System | Histamine (51-45-6) |
Safety information for Histamine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Histamine
| InChIKey | NTYJJOPFIAHURM-UHFFFAOYSA-N |
Histamine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Histamine, ≥97% CAS 51-45-6View Details
Histamine, ≥97% CAS 51-45-6View Details
51-45-6 -
 Histamine 98% CAS 51-45-6View Details
Histamine 98% CAS 51-45-6View Details
51-45-6 -
 Histamine CAS 51-45-6View Details
Histamine CAS 51-45-6View Details
51-45-6 -
 Histamine CAS 51-45-6View Details
Histamine CAS 51-45-6View Details
51-45-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
