Sodium tetrafluoroborate
Synonym(s):Sodium fluoroborate;Sodium tetrafluoroborate
- CAS NO.:13755-29-8
- Empirical Formula: BF4Na
- Molecular Weight: 109.79
- MDL number: MFCD00003515
- EINECS: 237-340-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
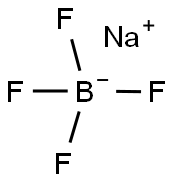
What is Sodium tetrafluoroborate?
Chemical properties
white crystals or powder
The Uses of Sodium tetrafluoroborate
Sodium Tetrafluoroborate is used in various metal finishing and plating circuits.
The Uses of Sodium tetrafluoroborate
Fluorinating agent, see Lawton, Levy, J. Am. Chem. Soc. 77, 6083 (1955).
The Uses of Sodium tetrafluoroborate
Sodium tetrafluoroborate may be used for the laboratory synthesis of boron fluoride. It may be used for the synthesis of fluoro-nucleic acids and fluoroheterocyclic compounds.
General Description
Sodium tetrafluoroborate (NaBF4) is colorless sodium salt and its crystals belong to the rhombic crystal system. It can be synthesized by reacting tetrafluoroboric acid with sodium carbonate or hydroxide.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of Fand Na2O.
Purification Methods
Crystallise the fluoroborate from hot water (50mL/g) by cooling to 0o. Alternatively, free it from insoluble material by dissolving it in a minimum amount of water; then fluoride ions are removed by adding concentrated lanthanum nitrate in excess. After removing lanthanum fluoride by centrifugation, the supernatant is passed through a cation-exchange column (Dowex 50, Na+-form) to remove any remaining lanthanum [Anbar & Guttman J Phys Chem 64 1896 1960]. It has also been recrystallised from anhydrous MeOH and dried in a vacuum at 70o for 16hours. Keep it dry as it is hygrosopic. [Delville et al. J Am Chem Soc 109 7293 1987.]
Properties of Sodium tetrafluoroborate
| Melting point: | 384 °C |
| Density | 2.47 g/mL at 25 °C(lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | water: soluble(lit.) |
| form | Powder, Crystals or Needles |
| color | White to yellow to beige to brown |
| Specific Gravity | 2.47 |
| Water Solubility | 108 g/100 mL (26 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8617 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Moisture sensitive; do not store in glass (though the completely dry material does not etch glass). Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 13755-29-8(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), tetrafluoro-, sodium (13755-29-8) |
Safety information for Sodium tetrafluoroborate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium tetrafluoroborate
Sodium tetrafluoroborate manufacturer
JSK Chemicals
Jay Intermediates And Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Sodium tetrafluoroborate 98%View Details
Sodium tetrafluoroborate 98%View Details -
 Sodium tetrafluoroborate CAS 13755-29-8View Details
Sodium tetrafluoroborate CAS 13755-29-8View Details
13755-29-8 -
 Sodium tetrafluoroborate CAS 13755-29-8View Details
Sodium tetrafluoroborate CAS 13755-29-8View Details
13755-29-8 -
 Sodium Fluoborate chemical, 99.9, 50 Kg BagView Details
Sodium Fluoborate chemical, 99.9, 50 Kg BagView Details
13755-29-8 -
 Sodium Fluoborate, 99.08%, 99.08%View Details
Sodium Fluoborate, 99.08%, 99.08%View Details
13755-29-8 -
 Powder Sodium Fluoroborate, Purity: 98% Min, Packaging Type: Hdpe BagView Details
Powder Sodium Fluoroborate, Purity: 98% Min, Packaging Type: Hdpe BagView Details
13755-29-8 -
 Sodium Fluoroborate ChemicalView Details
Sodium Fluoroborate ChemicalView Details
13755-29-8 -
 Sodium Tetra fluoroborate, 98%View Details
Sodium Tetra fluoroborate, 98%View Details
13755-29-8
