Sodium sulfide nonahydrate
Synonym(s):Disodium sulfide nonahydrate;Sodium Monosulfide;Sodium sulfide enneahydrate;Sodium Sulfide, Nonahydrate
- CAS NO.:1313-84-4
- Empirical Formula: H2Na2OS
- Molecular Weight: 96.06
- MDL number: MFCD00149183
- EINECS: 639-249-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
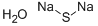
What is Sodium sulfide nonahydrate?
Chemical properties
colourless, white or yellow crystals with an odour of rotten eggs
The Uses of Sodium sulfide nonahydrate
Sodium sulfide nonahydrate has been used as a source of H2S, to study the effect of H2S on amyloid fibrils associated with neurodegenerative diseases. It has been used in the preparation of sulfide standards for the quantitative analysis of sulfides in raw wastewater (sewage) samples.
It serves as a catalyst during synthesis of thioamides.
The Uses of Sodium sulfide nonahydrate
sodium sulphide nonahydrate has the following uses:sulfur dyestuff; leather depilatory;rayon denitration;dyeing auxiliary;sodium sulphide;including crystallization water; sodium sulphide or sulfureT;dyeing auxiliary.
The Uses of Sodium sulfide nonahydrate
It is the precursor material for preparation of cadmium sulfide quantum dots. Also employed in the pulp and paper industry. It is likewise employed in the making of colors and dyestuffs. It is too useful in the trace quantities for the rapid synthesis of small silver nanocubes through mediating polyol reduction. It has as a significant role in breaking up the feather keratin useful for the preparation of bio-polymer.
What are the applications of Application
Sodium sulfide nonahydrate is a useful reagent for preparing surface functionalized cadmium sulfide quantum dots
General Description
Sodium sulfide nonahydrate participates in the conversion of nitroxides to amines, via reduction.
Purification Methods
Some purification of the hydrated salt can be achieved by selecting large crystals and removing the surface layer (contaminated with oxidation products) by washing with distilled water. Other metal ions can be removed from Na2S solutions by passage through a column of Dowex ion-exchange A-1 resin, Na+-form. The hydrated salt can be rendered anhydrous by heating it in a stream of H2 or N2 until water is no longer evolved. (The resulting cake should not be heated to fusion because it is readily oxidised.) Recrystallise it from distilled water [Anderson & Azowlay J Chem Soc, Dalton Trans 469 1986]. Note that sodium sulfide hydrolyses in H2O to form NaHS + H2O, and is therefore alkaline. A 0.1N solution in H2O is 86% hydrolysed at room temperature. Its solubility in H2O is 8% at 0o, 12% at 20o and 30% at 50o. The anhydrous salt is obtained by allowing it to stand in a vacuum over conc H2SO4 or P2O5 at 45o to start with, then at 30-35o when the salt contains 4% of water. The last traces of water are removed by heating to 700o in a glass or porcelain tube in a stream of H2 to give pure H2S. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 358-360 1963.]
Properties of Sodium sulfide nonahydrate
| Melting point: | 50 °C |
| Boiling point: | 920°C |
| Density | 1.427 |
| storage temp. | 2-8°C |
| solubility | Aqueous Acid (Slightly), Water (Sparingly) |
| form | Solid |
| color | colorless or slightly yellow |
| Specific Gravity | 1.427 |
| Water Solubility | 180 G/L (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8681 |
| Stability: | Stable. Hygroscopic. Light-sensitive. Flammable. Avoid exposure to air, acids, oxidizers, strong bases, strong reducing agents, most common metals. |
| CAS DataBase Reference | 1313-84-4(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium sulfide (Na2S), nonahydrate (1313-84-4) |
Safety information for Sodium sulfide nonahydrate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium sulfide nonahydrate
| InChIKey | GDQBTRYKLOBMLL-UHFFFAOYSA-N |
Sodium sulfide nonahydrate manufacturer
ALPHA CHEMIKA
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Sodium sulfide nonahydrate CAS 1313-84-4View Details
Sodium sulfide nonahydrate CAS 1313-84-4View Details
1313-84-4 -
 Sodium sulfide nonahydrate CAS 1313-84-4View Details
Sodium sulfide nonahydrate CAS 1313-84-4View Details
1313-84-4 -
 Sodium sulfide nonahydrate CAS 1313-84-4View Details
Sodium sulfide nonahydrate CAS 1313-84-4View Details
1313-84-4 -
 ACS Grade Sodium Sulfide Nonahydrate Cas 1313-84-4View Details
ACS Grade Sodium Sulfide Nonahydrate Cas 1313-84-4View Details
1313-84-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
