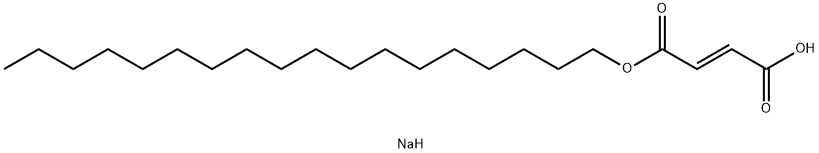
Sodium stearate
Synonym(s):Octadecanoic acid sodium salt;Stearic acid sodium salt
- CAS NO.:822-16-2
- Empirical Formula: C18H35NaO2
- Molecular Weight: 306.45907
- MDL number: MFCD00036404
- EINECS: 212-490-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-12 17:55:12

What is Sodium stearate?
Description
Sodium stearate is the sodium salt of stearic acid. This white solid is the most common soap. It is found in many types of solid deodorants, rubbers, latex paints, and inks. It is also a component of some food additives and food flavorings.
Description
Humans began to use cleaning substances that resemble modern soaps almost five millennia ago. Early crude soaps were made from natural fats and oils and available alkaline materials such as wood ashes. During the Industrial Revolution, manufacturers began to make more refined soaps from purified fatty acids and alkalis such as lye (sodium or potassium hydroxide), quicklime (calcium oxide), or slaked lime (calcium hydroxide).
Sodium stearate is the most common fatty acid salt in today’s soaps. Common sources of the starting material, stearic acid, are vegetable triglycerides obtained from coconut and palm oils and animal triglycerides from tallow. The names stearic and stearate are derived from stéar, the Greek word for tallow.
Besides being a major soap component, sodium stearate is used as an additive in other cosmetic products to form solid “stick” shapes. According to Acme-Hardesty, a Blue Bell, PA–based manufacturer of biobased products, sodium stearate has a wide range of additional uses, including
Many Web sites provide recipes for making soap at home. But all scratch recipes require lye, which is better off being handled in the lab. If you are willing not to make soap from scratch, you can purchase a “melt and pour” soap, in which the desired oil or fat is already treated with lye. Happy soapmaking!
Chemical properties
white to off-white powder
Chemical properties
Sodium stearate, NaC18H35O2, white solid, soluble, froth or foam upon shaking the water solution (soap), formed by reaction of NaOH and stearic acid (in alcoholic solution) and evaporating. Used as a source of stearate.
The Uses of Sodium stearate
sodium stearate is a fatty acid used as a waterproofing agent. Sodium stearate is one of the least allergy-causing sodium salts of fatty acids. It is non-irritating to the skin.
The Uses of Sodium stearate
β-Lactamase inhibitor
The Uses of Sodium stearate
Pharmaceutic aid (emulsifying and stiffening agent). In glycerol suppositories; also in toothpaste; as waterproofing agent.
The Uses of Sodium stearate
Sodium Stearate is the sodium salt of stearic acid. it functions as a binder, emulsifier, and anticaking agent. it is used as a plasticizer in chewing gum base.
The Uses of Sodium stearate
Characteristic of soaps, sodium stearate has both hydrophilic and hydrophobic parts, the carboxylate and the long hydrocarbon chain, respectively. These two chemically different components induce the formation of micelles, which present the hydrophilic heads outwards and their hydrophobic (hydrocarbon) tails inwards, providing a lipophilic environment for hydrophobic compounds. It is also used in the pharmaceutical industry as a surfactant to aid the solubility of hydrophobic compounds in the production of various mouth foams.
Definition
ChEBI: An organic sodium salt comprising equal numbers of sodium and stearate ions.
Production Methods
Sodium stearate is produced as a major component of soap upon saponification of oils and fats. The percentage of the sodium stearate depends on the ingredient fats. Tallow is especially high in stearic acid content (as the triglyceride), whereas most fats only contain a few percent. The idealized equation for the formation of sodium stearate from stearin (the triglyceride of stearic acid) follows :
(C18H35O2)3C3H5 → C3H5(OH)3 + 3 C17H35CO2Na
Purified sodium stearate can be made by neutralizing stearic acid with sodium hydroxide.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of Na2O.
Purification Methods
It is better to prepare it by adding a slight excess of octadecanoic acid to ethanolic NaOH, evaporating and extracting the residue with dry Et2O. [Beilstein 2 III 1003.]
Properties of Sodium stearate
| Melting point: | 270 °C |
| Density | 1.07 g/cm3 |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in water and in ethanol (96 per cent). |
| form | Powder |
| appearance | white solid or powder |
| color | white |
| Odor | wh. fine powd., fatty (tallow) odor |
| Water Solubility | SOLUBLE IN COLD AND HOT WATER |
| Merck | 14,8678 |
| BRN | 3576813 |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 3 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 822-16-2(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium stearate (822-16-2) |
Safety information for Sodium stearate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Sodium stearate
| InChIKey | RYYKJJJTJZKILX-UHFFFAOYSA-M |
Sodium stearate manufacturer
Vasa Pharmachem Pvt Ltd. (VPPL)
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 Sodium stearate 98%View Details
Sodium stearate 98%View Details -
 Sodium stearate CAS 822-16-2View Details
Sodium stearate CAS 822-16-2View Details
822-16-2 -
 Sodium stearate CAS 822-16-2View Details
Sodium stearate CAS 822-16-2View Details
822-16-2 -
 Sodium stearate CAS 822-16-2View Details
Sodium stearate CAS 822-16-2View Details
822-16-2 -
 Sodium stearate CAS 822-16-2View Details
Sodium stearate CAS 822-16-2View Details
822-16-2 -
 Sodium Stearate CAS 822-16-2View Details
Sodium Stearate CAS 822-16-2View Details
822-16-2 -
 Sodium stearate 95% CAS 822-16-2View Details
Sodium stearate 95% CAS 822-16-2View Details
822-16-2 -
 SODIUM STEARATE CAS 822-16-2View Details
SODIUM STEARATE CAS 822-16-2View Details
822-16-2
