Sodium hydrosulfide
- CAS NO.:16721-80-5
- Empirical Formula: HNaS
- Molecular Weight: 56.06
- MDL number: MFCD00011124
- EINECS: 240-778-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:27:30
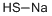
What is Sodium hydrosulfide?
Chemical properties
white crystals or powder with a very unpleasant smell
The Uses of Sodium hydrosulfide
the copper ore beneficiation,the sulfurous acid dyeing of manmade fibre,the synthesis of organic intermediates,the preparation of auxiliary agent of sulfur dyes,and waste water treatment; raw material to produce ammonium sulphate and ethanethiol
The Uses of Sodium hydrosulfide
In paper pulping, in dyestuffs processing, in rayon and cellophane desulfurizing, in unhairing hides and as a bleaching reagent Sodium hydrogen sulfide is used in cloth, paper, pulp and dyestuff industries. It acts as a floating agent in copper mining as well as to remove hairs from hides in leather industries. It is also used as an intermediate in synthetic chemistry. It is utilized as a raw material in the production of ammonium sulphate and ethanethiol.
General Description
Sodium hydrosulfide is a colorless to light yellow crystalline solid or fused mass. Sodium hydrosulfide is corrosive to skin and metal. Used in paper pulping, manufacturing dyes, and dehairing hides.
Air & Water Reactions
Soluble in water. The solution slowly evolves hydrogen sulfide gas. Crystals hydrolyze in moist air to sodium hydroxide and sodium sulfide. Liable to spontaneous heating in moist air.
Reactivity Profile
A chemical base. Reacts with acids to release flammable and toxic gaseous hydrogen sulfide. As long as the solution is kept strongly alkaline, pH > 10, there is very little release of H2S. At pH = 7, the percent concentration of H2S released is close to 80%.
Hazard
Contact with acids causes evolution of toxic gases.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Industrial uses
Sodium hydrosulfide (NaHS) is stable only in solution. When in contact with oxygen
(air), it slowly oxidizes. If a solution of NaHS is heated, it is converted into Na2S and H2S.
Sodium hydrosulfide can be used as a replacement for Na2S·9H2O during sulfidization
of oxide minerals. Sodium hydrosulfide in solution has a much lower alkalinity than
Na2S.
Although the performance of NaHS is not the same as for Na2S, it is used because of its
cost-effectiveness.
Safety Profile
Poison by intraperitoneal and subcutaneous routes. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. Flammable when exposed to heat or flame. Spontaneous combustion. Reacts violently with Qazonium salts. Readily yields HS. When heated to decomposition it emits toxic fumes of SO, and NazO. See also SULFIDES and MERCAPTANS.
Properties of Sodium hydrosulfide
| Melting point: | 55°C |
| Density | 1,79 g/cm3 |
| RTECS | WE1900000 |
| Flash point: | 90°C |
| solubility | soluble in H2O, EtOh, ethyl ether |
| form | Solid |
| color | Off-white |
| Water Solubility | 620 g/L (20 ºC) |
| Sensitive | Moisture Sensitive & Hygroscopic |
| Stability: | Stable, but hygroscopic. Flammable solid. May ignite in air. |
| CAS DataBase Reference | 16721-80-5(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium hydrosulfide (16721-80-5) |
Safety information for Sodium hydrosulfide
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H251:Self-heating substances and mixtures H302:Acute toxicity,oral H312:Acute toxicity,dermal H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Sodium hydrosulfide
Sodium hydrosulfide manufacturer
Laxmi Industries
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Sodium hydrogen sulfide 98%View Details
Sodium hydrogen sulfide 98%View Details -
 Sodium hydrogen sulfide CAS 16721-80-5View Details
Sodium hydrogen sulfide CAS 16721-80-5View Details
16721-80-5 -
 Sodium hydrosulphide, 30% CAS 16721-80-5View Details
Sodium hydrosulphide, 30% CAS 16721-80-5View Details
16721-80-5 -
 Sodium hydrosulphide 30% CAS 16721-80-5View Details
Sodium hydrosulphide 30% CAS 16721-80-5View Details
16721-80-5 -
 Liquid Sodium Hydrogen Sulphide, For Industrial, Grade Standard: Technical GradeView Details
Liquid Sodium Hydrogen Sulphide, For Industrial, Grade Standard: Technical GradeView Details
16721-80-5 -
 Sodium BisulphideView Details
Sodium BisulphideView Details
16721-80-5 -
 SODIUM HYDROGEN SULFIDE CAS Number: 207683-19-0View Details
SODIUM HYDROGEN SULFIDE CAS Number: 207683-19-0View Details
16721-80-5 -
 Sodium hydrosulfide CAS: 16721-80-5View Details
Sodium hydrosulfide CAS: 16721-80-5View Details
16721-80-5
