Sodium hydride
Synonym(s):Sodium hydride suspension
- CAS NO.:7646-69-7
- Empirical Formula: HNa
- Molecular Weight: 24
- MDL number: MFCD00003471
- EINECS: 231-587-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
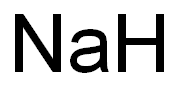
What is Sodium hydride?
Description
Sodium hydride, is a binary salt that has a specific hazard of releasing hydrogen upon contact with water. It is an odorless powder that is violently water reactive. The four-digit UN identification number is 1427. The NFPA 704 designation is health 3, flammability 3, and reactivity 2. The white space at the bottom of the diamond has a W with a slash through it, indicating water reactivity.
Description
Sodium hydride (NaH) is a strong base commonly used in organic chemistry to deprotonate alcohols, amine, amides, and other sufficiently acidic protons. Pure sodium hydride is prone to spontaneous ignition in moist air. For this reason, the most common form of sodium hydride used in labs is 60% sodium hydride dispersed on mineral oil.
Chemical properties
Grey solid. Pure sodium hydride forms colorless cubic crystals; however, the commercial product contains traces of sodium metal, giving it a light gray color. At atmospheric pressure, sodium hydride slowly evolves hydrogen above 300 ℃. At 420 ℃ decomposition is rapid but melting does not take place. Sodium hydride is a salt and therefore insoluble in inert organic solvents. It dissolves in molten sodium hydroxide, in sodium – potassium alloys and in molten LiCl – KCl eutectic mixtures (352 ℃). Sodium hydride is stable in dry air but ignites above 230 ℃, burning to form sodium oxide. It is hydrolyzed rapidly in moist air and as a dry powder it is spontaneously flammable. Sodium hydride reacts extremely violently with water, the heat of hydrolysis being sufficient to ignite the liberated hydrogen. It reacts with carbon dioxide to form sodium formate.
Physical properties
Silvery needles; refractive index 1.470; density 0.92 g/cm3; decomposes at 800°C; decomposes explosively in water; reacts violently with lower alcohols; dissolves in molten sodium and molten sodium hydroxide; insoluble in liquid ammonia, benzene, carbon tetrachloride and carbon disulfide. Sodium hydride is available commercially as 80 % and 60 % dispersions in mineral oil. The light-gray pourable material has an average particle size of 10 mm (Degussa, Chemetall, Ventron). The oil protects the surface of the highly reactive sodium hydride, which easily becomes passivated by the slightest atmospheric exposure or, worse, can ignite on longer contact with air.
The Uses of Sodium hydride
Sodium hydride is used to enhance the condensation reactions of carbonyl compounds through the Dieckmann condensation, Stobbe condensation, Darzens condensation and Claisen condensation. It acts as a reducing agent used to prepare diborane from boron trifluoride. It is also used in fuel cell vehicles. Further, it is used to dry some organic solvents. In addition to this, it is involved in the preparation of sulfur ylides, which is utilized for the conversion of ketones into epoxides.
The Uses of Sodium hydride
At low temperatures where reducing properties of sodium are undesirable as in the condensation of ketones and aldehydes with acid esters; in solution with molten sodium hydroxide for the reduction of oxide scale on metals; at high temperatures as a reducing agent and reduction catalyst.
The Uses of Sodium hydride
Direct intercalation into C60 results in the superconducting material (NaH)4C60.
Definition
sodium hydride: A white crystallinesolid, NaH; cubic; r.d. 0.92; decomposesabove 300°C (slow);completely decomposed at 800°C.Sodium hydride is prepared by thereaction of pure dry hydrogen withsodium at 350°C. Electrolysis ofsodium hydride in molten LiCl/KClleads to the evolution of hydrogen;this is taken as evidence for the ionicnature of NaH and the presence ofthe hydride ion (H–). It reacts violentlywith water to give sodium hydroxideand hydrogen, with halogensto give the halide and appropriatehydrogen halide, and ignites spontaneouslywith oxygen at 230°C. It is apowerful reducing agent with severallaboratory applications.
Production Methods
Sodium hydride, reactive with water yielding hydrogen gas and NaOH solution, formed by reaction of sodium and hydrogen at about 360 °C (680 °F). Used as a powerful reducing agent.
Reactions
The most important chemical property of sodium hydride is its basicity. Sodium hydride reacts irreversibly with a large number of compounds containing acidic hydrogen atoms, including alcohols, phenols, thiols, and amines, but also with weak carbon acids such as ketones, carboxylic esters, and acetylenic hydrocarbons. Sodium hydride is a poor reducing agent because of its high basicity and its insolubility in inert organic solvents. If the sodium hydride is complexed with alcoholates and metallic salts, complex reducing agents are formed that can reduce halides, alkenes, and ketones.
General Description
A silvery to whitish powder or slurry in oil. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Ignites or explodes in contact with air of high humidity [Bretherick 1979 p. 107]. Reacts violently with water producing a caustic solution (NaOH) and hydrogen (H2). Heat of reaction may ignite the hydrogen.
Reactivity Profile
Sodium hydride is a powerful reducing agent. Attacks SiO2 in glass. Ignites on contact with gaseous F2, Cl2, Br2, and I2 (the last at temperatures exceeding 100°C), especially in the presence of moisture, to form HF, HCl, HBr, and HI [Mellor 2:483 1946-47]. Reacts with sulfur to give Na2S and H2S [Bretherick 1979 p. 107]. Can react explosively with dimethyl sulfoxide [Chem. Eng. News 44(24):7 1966]. Reacts vigorously with acetylene, even at -60°C [Mellor 2:483 1946-47]. Spontaneously flammable in fluorine. Reaction with dimethylformamide, when heated, runs away [Chem. Eng. News, 1982, 60(28), 5]. Initiates a polymerization reaction in ethyl-2,2,3-trifluoropropionate such that the ester decomposed violently [Bretherick 5th ed. 1995]. Presence in the reaction of diethyl succinate and ethyl trifluoroacetate, has twice caused explosions [Chem. Brit., 1983, 19, 645].
Hazard
Dangerous fire risk, reacts violently with water evolving hydrogen. Irritant.
Health Hazard
SOLID: Will burn skin and eyes. Harmful if swallowed.
Fire Hazard
FLAMMABLE. MAY EXPLODE ON CONTACT WITH WATER. Accidental contact with water used to extinguish surrounding fire will result in the release of hydrogen gas and possible explosion.
Flammability and Explosibility
Highly flammable
Safety Profile
The powder ignites spontaneously in air. Flammable when exposed to heat or flame. Potentially explosive reaction with water, diethyl succinate + ethyltrifluoroacetate (above 60℃), dimethyl sulfoxide + heat, sulfur dioxide. Ignition or violent reaction with dimethylformamide (above 50℃), ethyl 2,2,3-trifluoropropionate, oxygen (at 230℃). Incompatible with acetylene + moisture, glycerin, halogens, sulphur. Normal fire extinguishers are unsuitable, use sand, ashes, solurn chloride. The commercial material may contain traces of sodium. When heated to decomposition it emits toxic fumes of Na2O. See also HYDRIDES.
Properties of Sodium hydride
| Melting point: | 800 °C (dec.) (lit.) |
| Density | 1.2 |
| Flash point: | 185°C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in molten sodium. Insoluble in ammonia, benzene,carbon tetrachloride, carbon disulfide and all organic solvents. |
| form | powder (moistened with oil) |
| appearance | Grey powder |
| color | White to pale gray |
| Water Solubility | REACTS |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,8625 |
| Exposure limits | ACGIH: TWA 5 mg/m3 OSHA: TWA 5 mg/m3 NIOSH: IDLH 2500 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 |
| CAS DataBase Reference | 7646-69-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium hydride(7646-69-7) |
| EPA Substance Registry System | Sodium hydride (NaH) (7646-69-7) |
Safety information for Sodium hydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H228:Flammable solids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium hydride
Sodium hydride manufacturer
JSK Chemicals
Chemit Laboratories
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Sodium hydride, 57-63% oil dispersion 99%View Details
Sodium hydride, 57-63% oil dispersion 99%View Details -
 Sodium hydride CAS 7646-69-7View Details
Sodium hydride CAS 7646-69-7View Details
7646-69-7 -
 Sodium Hydride, Packaging Type: Ms Drum, Packaging Size: 125 KgView Details
Sodium Hydride, Packaging Type: Ms Drum, Packaging Size: 125 KgView Details
7646-69-7 -
 SODIUM HYDRIDE, 98%, 25 Kg BagView Details
SODIUM HYDRIDE, 98%, 25 Kg BagView Details
7646-69-7 -
 Sodium Hydride, 67%, 25 kg drumView Details
Sodium Hydride, 67%, 25 kg drumView Details
7646-69-7 -
 SODIUM HYDRIDE 55-60%View Details
SODIUM HYDRIDE 55-60%View Details
7646-69-7 -
 Sodium Hydride PowderView Details
Sodium Hydride PowderView Details
7646-69-7 -
 Sodium Hydride, 25 Kg Bag, 98%View Details
Sodium Hydride, 25 Kg Bag, 98%View Details
7646-69-7
