Sodium formate
Synonym(s):E237;Formic acid sodium salt;Sodium formate
- CAS NO.:141-53-7
- Empirical Formula: CHNaO2
- Molecular Weight: 68.01
- MDL number: MFCD00013101
- EINECS: 205-488-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
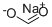
What is Sodium formate?
Description
Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.
Chemical properties
white crystals
Physical properties
White crystals; slightly hygroscopic; faint odor of formic acid; density 1.92 g/cm3; melts at 253°C; decomposes on further heating, first forming sodium oxalate and hydrogen and then sodium carbonate; very soluble in water; the aqueous solution neutral, pH about 7; soluble in glycerol; slightly soluble in alcohol; insoluble in ether.
Chemical properties
Sodium formate is usually appears as a white deliquescent powder. On heating, sodium formate decomposes to form sodium oxalate and hydrogen. The resulting sodium oxalate can be converted by further heating to sodium carbonate upon release of carbon monoxide.
The Uses of Sodium formate
Sodium formate is used in several fabric dyeing and printing processes. It is also used as a buffering agent for strong mineral acids to increase their pH, and as a food additive ( E237 ) .
The Uses of Sodium formate
Precipitant for noble metals.
The Uses of Sodium formate
In dyeing and printing fabrics; also In animal chemistry as a precipitant for the "noble" metals. Solubilizes trivalent metal ions in solution by forming complex ions. Buffering action adjusts the pH of strong mineral acids to higher values.
Definition
ChEBI: An organic sodium salt which is the monosodium salt of formic acid.
Preparation
Sodium formate can be prepared in the laboratory by neutralizing formic acid with sodium carbonate. It can also be obtained by reacting chloroform with an alcoholic solution of sodium hydroxide.
CHCl3 + 4NaOH → HCOONa + 3NaCl + 2H2O
or by reacting sodium hydroxide with chloral hydrate.
C2HCl3(OH)2 + NaOH → CHCl3 + HCOONa + H2O
The latter method is, in general, preferred to the former because the low aqueous solubility of CHCl3 makes it easier to separate out from the sodium formate solution, by fractional crystallization, than the soluble NaCl would be.
For commercial use, sodium formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 160 °C.
CO + NaOH → HCOONa
Sodium formate may also be created via the haloform reaction between ethanol and sodium hypochlorite in the presence of a base.
Preparation
Sodium formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 130°C and 6-8 bar pressure:CO + NaOH → HCO2Na
Crystal Structure
Sodium formate crystallizes in a monoclinic crystal system with the lattice parameters a = 6,19 ?, b = 6,72 ?, c = 6,49 ? and β = 121,7°.
General Description
Sodium formate is the colorless sodium salt of formic acid. It can be prepared by reacting formic acid with sodium hydroxide or carbonate. Its crystal structure has been investigated. Its crystals exhibit monoclinic-holohedral symmetry.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by ingestion, intravenous, and subcutaneous routes. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of NazO. See also FORMIC ACID.
Purification Methods
A saturated aqueous solution at 90o (0.8mL water/g) is filtered and allowed to cool slowly. (The final temperature should be above 30o to prevent formation of the hydrate.) After two such crystallissations, the crystals are dried in an oven at 130o, then under high vacuum. [Westrum et al. J Phys Chem 64 1553 1960, Roecker & Meyer J Am Chem Soc 108 4066 1986.] The salt has also been recrystallised twice from 1mM DTPA (diethylenetriaminepentaacetic acid, which was recrystallised 4x from MilliQ water and dried in a vacuum), then twice from water [Bielski & Thomas J Am Chem Soc 109 7761 1987]. [Beilstein 2 IV 3.]
Properties of Sodium formate
| Melting point: | 259-262 °C (lit.) |
| Boiling point: | 360 °C |
| Density | 1.16 g/mL at 20 °C |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 8 M at 20 °C, clear, colorless |
| form | Crystalline Powder |
| pka | 3.86[at 20 ℃] |
| Specific Gravity | 1.92 |
| color | White to off-white |
| PH | 7.4(1 mM solution);7.83(10 mM solution);8.25(100 mM solution);8.57(1000 mM solution) |
| Odor | Slight formic acid odour |
| PH Range | 7 - 8.5 |
| Water Solubility | Soluble |
| λmax | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.01 |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Sensitive | Hygroscopic |
| Merck | 14,8621 |
| BRN | 3595134 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. |
| CAS DataBase Reference | 141-53-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium methanoate(141-53-7) |
| EPA Substance Registry System | Sodium formate (141-53-7) |
Safety information for Sodium formate
Computed Descriptors for Sodium formate
Sodium formate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Sodium formate 98%View Details
Sodium formate 98%View Details -
 Sodium formate, For analysis ACS CAS 141-53-7View Details
Sodium formate, For analysis ACS CAS 141-53-7View Details
141-53-7 -
 Sodium Formate extrapure AR CAS 141-53-7View Details
Sodium Formate extrapure AR CAS 141-53-7View Details
141-53-7 -
 Sodium Formate pure CAS 141-53-7View Details
Sodium Formate pure CAS 141-53-7View Details
141-53-7 -
 Sodium FormateView Details
Sodium FormateView Details
141-53-7 -
 Sodium Formate ChemicalView Details
Sodium Formate ChemicalView Details
141-53-7 -
 Sodium FormateView Details
Sodium FormateView Details
141-53-7 -
 Sodium FormateView Details
Sodium FormateView Details
141-53-7
