Sodium dodecyl sulfate
Synonym(s):SDS;Sodium lauryl sulfate;Dodecyl sodium sulfate;Dodecyl sulfate sodium salt;Lauryl sulfate sodium salt
- CAS NO.:151-21-3
- Empirical Formula: C12H26O4S.Na
- Molecular Weight: 289.39
- MDL number: MFCD00036175
- EINECS: 205-788-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-28 08:51:53
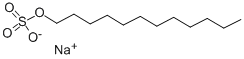
What is Sodium dodecyl sulfate?
Description
Sodium dodecyl sulfate (SDS), also known as Sodium lauryl sulfate (SLS) is an anionic surfactant naturally derived from coconut or palm kernel oil. It typically consists of a mixture of sodium alkyl sulfates, primarily lauryl. SLS is used in shampoos, toothpastes, facial cleansers, and many other toiletries. This amphiphilic molecule provides foaming and degreasing properties to these products. It is also used in creams and pastes in order to properly disperse ingredients and as a research tool in protein biochemistry. SLS also has some bactericidal activity.
Description
Sodium dodecyl sulfate is an anionic surfactant, and is a typical representative of sulphate-based surfactant. It is abbreviated as SDS, and also known as AS, K12, coco alcohol sulfate, sodium lauryl sulfate and foaming agent. The commercial products are usually white to light yellow crystalline powder.
Sodium dodecyl sulfate is a major component of detergent. It is usually used in the DNA extraction process to separate DNA after protein denaturation.
Chemical properties
Sodium lauryl sulfate consists of white or cream to pale yellow coloured crystals, flakes, or powder having a smooth feel, a soapy, bitter taste, and a faint odor of fatty substances. It is easily soluble in water.
The Uses of Sodium dodecyl sulfate
Sodium dodecyl sulfate is an emulsifier and whipping aid that has a solubility of 1 g in 10 ml of water. It functions as an emulsifier in egg whites. It is used as a whipping aid in marshmallows and angel food cake mix. It also functions to aid in dissolving fumaric acid.
What are the applications of Application
Sodium dodecyl sulfate is an anionic detergent and protein denaturant. It can be used for the following applications:
(1) SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
(2) Chromatin immunoprecipitation
(3) as a component of radioimmunoprecipitation assay buffer for immunoblotting
(4) As an anionic detergent
Production Methods
Sodium lauryl sulfate is prepared by sulfation of lauryl alcohol, followed by neutralization with sodium carbonate.
What are the applications of Application
Sodium dodecyl sulfate has excellent detergency, emulsification and foaming power, can be used as detergents and textile auxiliaries, and is also used as an anionic surfactant, toothpaste foaming agent, mine fire extinguishing agents, foaming agents for fire extinguishers, emulsion polymerization emulsifiers, emulsifying and dispersing agents for medical use, shampoo and other cosmetic products, wool detergent, detergent for silky class fine fabrics and flotation agent for metal beneficiation.
GB 2760-96 stipulates as processing aids for food industry. It used as foaming agents; emulsifying agents; and anionic surfactants. It is used for cakes, drinks, proteins, fruits, fruit juice, and edible oil, and so on.
Preparation
Sodium dodecyl sulfate can be synthesized by reacting dodecyl alcohol with sulfur trioxide gas, followed by neutralization with sodium hydroxide. The preparation of SDS involves the following steps: The reaction takes place in a vertical reactor at 32 °C. Nitrogen gas is introduced through the gas vents at a flow rate of 85.9 L/min. Lauryl alcohol is added at a flow rate of 58 g/min at 82.7 kPa. Liquid sulfur trioxide is fed into the flash evaporator at 124.1 kPa, with a flow rate of 0.9072 kg/h and a flash temperature of 100 °C. The sulfated product is quickly cooled to 50 °C, aged for 10-20 min, then neutralized with a base in a neutralization kettle controlled at 50 °C. The pH is adjusted to 7-8.5, and the liquid product is spray dried to obtain a solid product.
Pharmaceutical Applications
Sodium lauryl sulfate is an anionic surfactant employed in a wide
range of nonparenteral pharmaceutical formulations and cosmetics.
It is a detergent and wetting agent effective in both alkaline and
acidic conditions. In recent years it has found application in
analytical electrophoretic techniques: SDS (sodium dodecyl sulfate)
polyacrylamide gel electrophoresis is one of the more widely used
techniques for the analysis of proteins; and sodium lauryl sulfate
has been used to enhance the selectivity of micellar electrokinetic
chromatography (MEKC).
Biochem/physiol Actions
Sodium dodecyl sulphate?(SDS) helps to quickly disrupt the biological membranes. It is usually used as one of the major constituent of several reagents, that are used to purify nucleic acids. SDS can block the activity of RNase and deoxyribonuclease (DNase).
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. An experimental teratogen. A human skin irritant. An experimental eye and severe skin irritant. A mild allergen. Mutation data reported. When heated to decomposition it emits toxic fumes of SO, and Na2O. See also ESTERS and SULFATES.
Safety
Sodium lauryl sulfate is a moderately toxic material with acute toxic effects including irritation to the skin, eyes, mucous membranes, upper respiratory tract, and stomach. Repeated, prolonged exposure to dilute solutions may cause drying and cracking of the skin; contact dermatitis may develop. Prolonged inhalation of sodium lauryl sulfate will damage the lungs. Pulmonary sensitization is possible, resulting in hyperactive airway dysfunction and pulmonary allergy.
Storage
Sodium lauryl sulfate is stable under normal storage conditions.
However, in solution, under extreme conditions, i.e. pH 2.5 or
below, it undergoes hydrolysis to lauryl alcohol and sodium
bisulfate.
The bulk material should be stored in a well-closed container
away from strong oxidizing agents in a cool, dry place.
Properties and Applications
|
INDEX/GRADE |
SPECIFICATION |
|
Appearance |
Needle form without hard lumps |
|
Active matter |
92% min |
|
Petroleum eth soluble substances |
1.5% max |
|
Inorganic salts (NaCl+Na 2 SO 4 ) |
5.5% max |
|
Water |
5.0% max |
|
pH value (1% aq. solution) |
7.5-9.5 |
|
Whiteness (WG) |
80 min |
|
Heavy metal |
20ppm max |
|
As |
3ppm max |
Incompatibilities
Sodium lauryl sulfate reacts with cationic surfactants, causing loss
of activity even in concentrations too low to cause precipitation.
Unlike soaps, it is compatible with dilute acids and calcium and
magnesium ions.
Sodium lauryl sulfate is incompatible with salts of polyvalent
metal ions, such as aluminum, lead, tin or zinc, and precipitates with
potassium salts.
Properties of Sodium dodecyl sulfate
| Melting point: | 204-207 °C (lit.) |
| Density | 1.03 g/mL at 20 °C |
| FEMA | 4437 | SODIUM LAURYL SULFATE |
| Flash point: | >100°C |
| storage temp. | 2-8°C |
| solubility | H2O: 0.1 M, clear to nearly clear, colorless to slightly yellow |
| form | Powder or Crystals |
| color | White to pale yellow |
| PH Range | 7.2 |
| Odor | Slight fatty odour |
| PH | 6-9 (10g/l, H2O, 20℃) |
| Water Solubility | ca. 150 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: 0.3 λ: 280 nm Amax: 0.2 |
| Merck | 14,8636 |
| BRN | 3599286 |
| CAS DataBase Reference | 151-21-3(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium lauryl sulfate (151-21-3) |
Safety information for Sodium dodecyl sulfate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium dodecyl sulfate
| InChIKey | DBMJMQXJHONAFJ-UHFFFAOYSA-M |
Sodium dodecyl sulfate manufacturer
JSK Chemicals
C.H. Chemicals
Pharma Links
Bazayan & Co.
Attar Global
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 151 -21 -3 Sodium Lauryl Sulphate 98%View Details
151 -21 -3 Sodium Lauryl Sulphate 98%View Details
151 -21 -3 -
 Sodium dodecyl sulfate 99%View Details
Sodium dodecyl sulfate 99%View Details -
 Sodium dodecyl sulfate 98%View Details
Sodium dodecyl sulfate 98%View Details -
 Sodium dodecyl sulfate 98%View Details
Sodium dodecyl sulfate 98%View Details -
 Tris-Glycine-Sds Buffer CAS 151-21-3View Details
Tris-Glycine-Sds Buffer CAS 151-21-3View Details
151-21-3 -
 Sodium n-dodecyl sulfate CAS 151-21-3View Details
Sodium n-dodecyl sulfate CAS 151-21-3View Details
151-21-3 -
 Sodium Dodecylsulfate CAS 151-21-3View Details
Sodium Dodecylsulfate CAS 151-21-3View Details
151-21-3 -
 Sodium Dodecyl Sulfate (sds) CASView Details
Sodium Dodecyl Sulfate (sds) CASView Details
