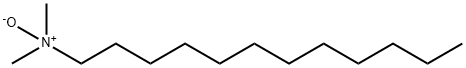
Ammonium lauryl sulfate
Synonym(s):Ammonium dodecyl sulfate;Dodecyl sulfate ammonium salt
- CAS NO.:2235-54-3
- Empirical Formula: C12H26O4S.H3N
- Molecular Weight: 283.43
- MDL number: MFCD00050675
- EINECS: 218-793-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
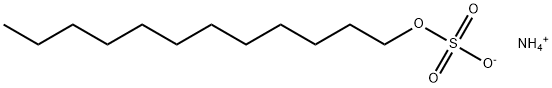
What is Ammonium lauryl sulfate?
Chemical properties
yellow viscous liquid
The Uses of Ammonium lauryl sulfate
Ammonium lauryl sulfate is an anionic surfactant which can be used in the preparation of porous building ceramics by gelcasting and formulation of cosmetic products. It can also be used as a corrosion inhibitor for carbon steel in acidic solution.
The Uses of Ammonium lauryl sulfate
ammonium lauryl sulfate is a surfactant with emulsifying capabilities. given its detergent properties, at mild acidic pH levels it can be used as an anionic surfactant cleanser. It is considered one of the most irritating surfactants, causing dryness and skin redness. Today, it is either combined with anti-irritant ingredients to reduce sensitivity or replaced with a less irritating but similar surfactant, such as ammonium laureth sulfate.
General Description
Light yellow liquid. May float or sink and mix with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic inorganic salts, such as Ammonium lauryl sulfate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid.
Health Hazard
Contact with liquid irritates eyes and may have drying effect on the skin. Prolonged contact will cause skin irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen and sulfur may form in fires.
Purification Methods
Recrystallise it first from 90% EtOH and then twice from absolute EtOH, and finally dry it in a vacuum. [Beilstein 1 III 1786.]
Properties of Ammonium lauryl sulfate
| Density | 1.02 g/mL at 20 °C |
| refractive index | n |
| Flash point: | 110 °C |
| storage temp. | 15-25°C |
| BRN | 5188379 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C12H26O4S.H3N/c1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15;/h2-12H2,1H3,(H,13,14,15);1H3 |
| CAS DataBase Reference | 2235-54-3(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium lauryl sulfate (2235-54-3) |
Safety information for Ammonium lauryl sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Ammonium lauryl sulfate
| InChIKey | BTBJBAZGXNKLQC-UHFFFAOYSA-N |
| SMILES | [NH4+].S([O-])(OCCCCCCCCCCCC)(=O)=O |
Ammonium lauryl sulfate manufacturer
Alpha Chemicals Pvt Ltd
Vinamax Organics Pvt Ltd
Jeetchem Organics Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Ammonium dodecyl sulfate 98%View Details
Ammonium dodecyl sulfate 98%View Details -
 2235-54-3 98%View Details
2235-54-3 98%View Details
2235-54-3 -
 Ammonium dodecyl sulfate 99%View Details
Ammonium dodecyl sulfate 99%View Details
2235-54-3 -
 Ammonium dodecyl sulfate 99%View Details
Ammonium dodecyl sulfate 99%View Details
2235-54-3 -
 Ammonium lauryl sulfate solution CAS 2235-54-3View Details
Ammonium lauryl sulfate solution CAS 2235-54-3View Details
2235-54-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1