Sodium dodecylbenzenesulphonate
Synonym(s):Dodecylbenzenesulfonic acid sodium salt;SDBS
- CAS NO.:25155-30-0
- Empirical Formula: C18H29NaO3S
- Molecular Weight: 348.48
- MDL number: MFCD00011508
- EINECS: 246-680-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:47
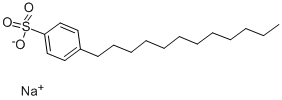
What is Sodium dodecylbenzenesulphonate?
Description
Sodium dodecyl benzene sulfonate is a series of organic compounds with the formula C12H25C6H4SO3Na. It is a colourless salt with useful properties as a surfactant. It is usually produced as a mixture of related sulfonates. It is a major component of laundry detergent.
Chemical properties
white or light yellow flakes
The Uses of Sodium dodecylbenzenesulphonate
A surfactant used in proteomics research.
The Uses of Sodium dodecylbenzenesulphonate
Anionic detergent.
The Uses of Sodium dodecylbenzenesulphonate
Sodium dodecylbenzenesulfonate has been used to stabilize dispersions of graphene nanoflakes (GNFs) during the preparation of the liquid phase of GNFs. It can also suspend single-walled carbon nanotubes as individuals in aqueous media and also give well-resolved spectral features.
What are the applications of Application
Sodium dodecylbenzenesulfonate is a surfactant used in proteomics research
General Description
Sodium dodecylbenzenesulfonate is a white to light yellow flakes, granules or powder. Sodium dodecylbenzenesulphonate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Sodium dodecylbenzenesulphonate is used as a synthetic detergent.
Air & Water Reactions
Sodium dodecylbenzenesulphonate is soluble in water.
Reactivity Profile
SODIUM DODECYLBENZENESULFONATE is incompatible with strong oxidizers.
Health Hazard
Minor skin and eye irritant. INGESTION: May cause vomiting, diarrhea, and intestinal distension.
Flammability and Explosibility
Non flammable
Industrial uses
These frothers are mixtures of alcohols containing 6–8 carbon atoms. They were at one
time marketed by DuPont and they are tailored frothers for specific ore types. The bestknown
frother from this group is methyl isobutyl carbinol (MIBC) and 2-ethyl hexanol.
Aliphatic alcohol frothers are used as mixtures of different carbon lengths and as a
mixture of hydrocarbon oils.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. A skin and severe eye irritant. When heated to decomposiuon it emits tomc fumes of NazO. See also SULFONATES
Synthesis
Sodium dodecylbenzenesulphonate is synthesised using 2-phenyldodecane as raw material by chemical reaction. The specific synthesis steps are as follows:
To a 5 L round bottom flask equipped with mechanical stirrer and an addition funnel was added octadecane and 2-phenyldodecane mixture ( 1.73 kg, 28%> 2-phenyldodecane). The reaction mixture was sparged with argon, warmed to 35°C and 1.25 weight % of oleum (632 g, 1.58 mol) was added dropwise, via addition funnel, to reaction mixture. The reaction mixture was stirred for 1.5 hours at room temperature. Upon completion, the reaction mixture was heated to 50°C and transferred to a separatory funnel and allowed to separate. The bottom layer was added slowly to a stirred solution of 15%> NaOH (aq) (2 L) at 10°C. Upon complete addition the resulting suspension was stirred for an additional 60 minutes. The solid was subsequently isolated by filtration and washed twice with ice-cold water. The solids were air dried for 16 hours and vacuum dried at 80°C to yield sodium, 4-(dodecan-2-yl) benzenesulfonate (555 g, 80.8% yield, 98.5% purity). lH NMR (400 MHz, (CD3)2S0/CDC13) δ 0.84 (t, J = 7.0 Hz, 3H), 0.95- 1.38 (m, 19H), 1.51 (pquart, J = 7.3 Hz, 2H), 2.65 (psext, J = 7.0 Hz, 1 H), 7.1 1 (d, J = 7.6 Hz, 2H), 7.56 (d, J = 8.4 Hz, 2H). 13C NMR (101 MHz, (CD3)2S0/CDC13) δ 13.8, 22.0, 22.1 , 27.0, 28.9, 28.9, 28.9, 28.9, 31.2, 37.6, 38.8, 125.4, 125.8, 145.3, 147.7.
Purification Methods
It crystallises from propan-2-ol or H2O. [Gray et al. J Org Chem 20 515 1955, Beilstein 11 IV 514.]
Alkyl benzene sulfonates
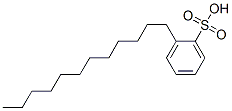
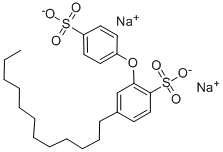
Most sodium dodecyl benzene sulfonates are a member of the linear alkyl benzene sulfonates, meaning that the dodecyl group (C12H25) is un branched. This dodecyl chain is attached at the 4- position of the benzene sulfonate group. Linear dodecyl-4-benzene sulfonate anions can exist in six isomers (ignoring optical isomers), depending on the carbon of the dodecyl group that is attached to the benzene ring. The isomer shown below left is 4-(5-dodecyl ) benzene sulfonate (4 indicating the position of the benzene ring, 5 indicating the position on the dodecane chain). Branched isomers, e.g. those derived from tetramerized propylene, are also known (below right) but are not as widely used because they biodegrade too slowly.
Production
Trillions of kilograms are produced annually. Given the large scale of the application, the alkyl benzene sulfonates have been prepared by many methods. In the most common route, benzene is alkylated by long chain mono alkenes (e.g. dodecene) using hydrogen fluoride as a catalyst. The purified dodecyl benzenes (and related derivatives) are then sulfonated with sulfur trioxide to give the sulfonic acid. The sulfonic acid is subsequently neutralized with sodium hydroxide.
Environmental considerations
Biodegradability has been well studied , and is affected by the isomerization (branching). The salt has an LD50 of 2.3 mg / liter for fish , about 4x more toxic than the branched tetra propylene benzene sulfonate. It is however biodegraded more rapidly. Oxidative degradation initiates at the alkyl chain.
Properties of Sodium dodecylbenzenesulphonate
| Melting point: | >300 °C |
| Boiling point: | 660.62℃[at 101 325 Pa] |
| Density | 1.02 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| pka | 0.7[at 20 ℃] |
| form | powder |
| color | light yellow |
| Water Solubility | 800mg/L at 25℃ |
| Merck | 14,8612 |
| BRN | 4171051 |
| Stability: | Stable. |
| CAS DataBase Reference | 25155-30-0(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium dodecylbenzenesulfonate (25155-30-0) |
Safety information for Sodium dodecylbenzenesulphonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium dodecylbenzenesulphonate
| InChIKey | JHJUUEHSAZXEEO-UHFFFAOYSA-M |
Sodium dodecylbenzenesulphonate manufacturer
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Sodium Dodecylbenzenesulfonate (SDBS) pure CAS 25155-30-0View Details
Sodium Dodecylbenzenesulfonate (SDBS) pure CAS 25155-30-0View Details
25155-30-0 -
 Dodecylbenzenesulphonic acid sodium salt CAS 25155-30-0View Details
Dodecylbenzenesulphonic acid sodium salt CAS 25155-30-0View Details
25155-30-0 -
 Sodium Dodecylbenzenesulfonate (SDBS) 80% CAS 25155-30-0View Details
Sodium Dodecylbenzenesulfonate (SDBS) 80% CAS 25155-30-0View Details
25155-30-0 -
 DODECYL BENZENE SULPHONIC ACID SODIUM SALT Purified CAS 25155-30-0View Details
DODECYL BENZENE SULPHONIC ACID SODIUM SALT Purified CAS 25155-30-0View Details
25155-30-0 -
 Sodium dodecylbenzenesulfonate 80% pure CAS 25155-30-0View Details
Sodium dodecylbenzenesulfonate 80% pure CAS 25155-30-0View Details
25155-30-0 -
 Dodecyl benzene sulphonic acid sodium salt 80% CAS 25155-30-0View Details
Dodecyl benzene sulphonic acid sodium salt 80% CAS 25155-30-0View Details
25155-30-0 -
 Sodium dodecylbenzenesulfonate CAS 25155-30-0View Details
Sodium dodecylbenzenesulfonate CAS 25155-30-0View Details
25155-30-0 -
 Sodium dodecylbenzenesulfonate CAS 25155-30-0View Details
Sodium dodecylbenzenesulfonate CAS 25155-30-0View Details
25155-30-0
