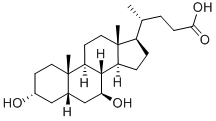
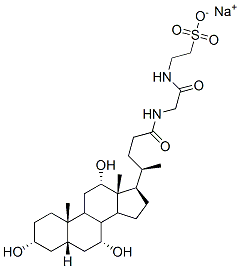
Sodium deoxycholate
Synonym(s):3α,12α-Dihydroxy-5β-cholanic acid sodium salt;7-Deoxycholic acid sodium salt;Deoxycholic acid sodium salt, Desoxycholic acid sodium salt ;Desoxycholic acid sodium salt;Sodium deoxycholate
- CAS NO.:302-95-4
- Empirical Formula: C24H41NaO4
- Molecular Weight: 416.58
- MDL number: MFCD00064139
- EINECS: 206-132-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
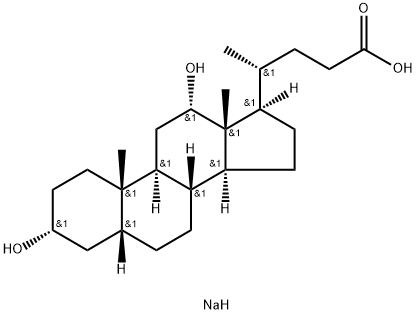
What is Sodium deoxycholate?
Chemical properties
Sodium deoxycholate is white to cream crystalline powder
The Uses of Sodium deoxycholate
Sodium deoxycholate is used as a component in cell lysis buffers.
The Uses of Sodium deoxycholate
Sodium deoxycholate is an ionic detergent for the solubilization of membrane-bound proteins.
The Uses of Sodium deoxycholate
Sodium deoxycholate is used as a choleretic and an anionic detergent. It is useful for the extraction of membrane receptors, plasma membrane proteins, nuclei isolation and solubilizes fats as well as membrane components. It acts as an intermediate for the production of corticosteroids and microbiological diagnostic media. In addition, it acts as a catalyst in the preparation of protein extracts for immunoblotting and immunoprecipitation of radiolabeled proteins.
What are the applications of Application
Deoxycholic acid sodium salt is an ionic detergent for the solubilization of membrane-bound proteins
Definition
ChEBI: Sodium deoxycholate is a bile acid salt. It contains a deoxycholate.
General Description
Sodium deoxycholate is a bile salt and an ionic detergent. Bile salts work along with lipids/fats/cholesterol and form mixed micelles in the intestine. These micelles help in fat digestion and absorption through the intestinal wall.
Biological Activity
Sodium deoxycholate (SDC) is an anionic detergent which has been used both to destroy infectious viruses and to fractionate viral components. Sodium deoxycholate begins to bind to the virus at less than 0.1 mM free equilibrium concentration. It causes lysis of the viral membrane at 0.9 +/- 0.1 mM free equilibrium concentration when 2.2 +/- 0.2 - 103 mol of sodium deoxycholate are bound per mol of the virus. The liberation of proteins from the membrane begins at 1.5 +/- 0.1 mM sodium deoxycholate, and the proteins released are virtually free from phospholipids above 2.0 mM sodium deoxycholate. The overall mechanism of sodium deoxycholate solubilization of the viral membrane resembles that of Triton X-100 and sodium dodecyl sulphate, except that with sodium deoxycholate, the various stages of membrane disruption occur at about 10-fold higher equilibrium free detergent concentrations. At sodium deoxycholate concentrations higher than 2.3 mM, the viral spike glycoproteins can be separated by sucrose gradient centrifugation or gel filtration into constituent polypeptides E1, E2 and E3[1-2].
Biochem/physiol Actions
Solubilizes fats for absorption in the intestines.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NasO.
Purification Methods
Recrystallise it from EtOH and dry it in an oven at 100o. The solution is freed from soluble components by repeated extraction with acid-washed charcoal. [Beilstein 10 IV 1608.]
References
[1] A Helenius. “Solubilization of the Semliki Forest virus membrane with sodium deoxycholate.” Biochimica et biophysica acta 436 2 (1976): 319–34.
[2] A E Auletta, M L Marlowe. “Effect of sodium deoxycholate on rubella virus.” Applied microbiology 16 10 (1968): 1624.
Properties of Sodium deoxycholate
| Melting point: | 357-365 °C |
| alpha | 44 º (c=2, H2O) |
| Density | 1.012 at 20.07℃ |
| vapor pressure | 0Pa at 20℃ |
| Flash point: | >110°C (own results) |
| storage temp. | room temp |
| solubility | water: soluble1 gm in 10 ml, clear, colorless to very faintly yellow |
| form | Powder |
| color | Yellow-tan |
| Odor | Odorless |
| PH | 7.5-9.0 (20g/l, H2O, 20℃) |
| PH Range | 7 - 10 |
| optical activity | [α]20/D +44±2°, c = 2% in H2O |
| Water Solubility | 330 g/L (15 ºC) |
| BRN | 3581950 |
| CAS DataBase Reference | 302-95-4(CAS DataBase Reference) |
| EPA Substance Registry System | Cholan-24-oic acid, 3,12-dihydroxy-, monosodium salt, (3.alpha.,5.beta.,12.alpha.)- (302-95-4) |
Safety information for Sodium deoxycholate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Sodium deoxycholate
Sodium deoxycholate manufacturer
Techno Pharmchem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 SODIUM DEOXYCHOLATE 302-95-4 99%View Details
SODIUM DEOXYCHOLATE 302-95-4 99%View Details
302-95-4 -
![Sodium Deoxycholate [for Electrophoresis] CAS 302-95-4](https://img.chemicalbook.in//Content/image/CP5.jpg) Sodium Deoxycholate [for Electrophoresis] CAS 302-95-4View Details
Sodium Deoxycholate [for Electrophoresis] CAS 302-95-4View Details
302-95-4 -
 Sodium deoxycholate 98% CAS 302-95-4View Details
Sodium deoxycholate 98% CAS 302-95-4View Details
302-95-4 -
 Sodium deoxycholate CAS 302-95-4View Details
Sodium deoxycholate CAS 302-95-4View Details
302-95-4 -
 Sodium deoxycholate CAS 302-95-4View Details
Sodium deoxycholate CAS 302-95-4View Details
302-95-4 -
 SODIUM DEOXYCHOLATE Extra Pure CAS 302-95-4View Details
SODIUM DEOXYCHOLATE Extra Pure CAS 302-95-4View Details
302-95-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
