Sodium cyanide
Synonym(s):Sodium cyanide
- CAS NO.:143-33-9
- Empirical Formula: CNNa
- Molecular Weight: 49.01
- MDL number: MFCD00003523
- EINECS: 205-599-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Sodium cyanide?
Description
Sodium cyanide, NaCN, is a cyanide salt that is a white, deliquescent, crystalline powder and is soluble in water. The specific gravity is 1.6, which is heavier than water. Sodium cyanide is toxic by inhalation and ingestion, with a TLV of 4.7 ppm and 5 mg/m3 of air. The target organs are the cardiovascular system, central nervous system, kidneys, liver, and skin. Reactions with acids can release flammable and toxic hydrogen cyanide gas. Cyanides are incompatible with all acids. The four-digit UN identification number is 1689.
The NFPA 704 designation is health 3, flammability 0, and reactivity 0. The primary uses are in gold and silver extraction from ores, electroplating, fumigation, and insecticides.
Chemical properties
Sodium cyanide is found as white granules, flakes or lumps. Sodium cyanide is shipped as pellets or briquettes. Odorless when dry. It absorbs water from air (is hygroscopic or deliquescent). Hydrogen cyanide gas released by sodium cyanide has a distinctive mild, bitter almond odor, but a large proportion of people cannot detect it; the odor does not provide adequate warning of hazardous concentrations.
Physical properties
Physical Properties White cubic crystals; hygroscopic; density 1.6 g/cm3; melts at 563°C; very soluble in water; aqueous solution strongly alkaline and decomposes rapidly.
The Uses of Sodium cyanide
Sodium cyanide is used for electroplating metals such as zinc, copper, cadmium, silver, and gold, and their alloys; for extracting gold and silver from ores; and as a fumigant and a chelating agent. It occurs in many varieties of maniocs (cassava), especially in bitter manioc.
The Uses of Sodium cyanide

A solution of the SM (0.42 g, 1.09 mmol) and NaCN (0.085 g, 1.73 mmol) in DMF (3 mL) was stirred at 100 C for 2 h. The reaction mixture was cooled to RT, diluted with DCM (20 mL), and washed with H2O (25 mL). The aq layer was further extracted with DCM (2 x 20 mL). The combined organics were dried (Na2SO4), concentrated, and purified by column chromatography (0-10% MeOH/DCM) to provide the product as a light brown oil which was used in the next step without further purification.
The Uses of Sodium cyanide
Extracting gold and silver from ores; electroplating baths; case hardening steel by liquid nitriding; manufacture of hydrocyanic acid and other cyanides.
The Uses of Sodium cyanide
Sodium cyanide is used as a starting material for the preparation of Reissert compounds, cyanogen bromide, cyanuric chloride and cyanogen chloride. It acts as a catalyst for the aminolysis of esters to primary amides. Furthermore, it is used for fumigation, in electroplating and for extracting gold and silver in mining industry. In organic synthesis, it is involved in the cyanation reaction of alkyl halides under phase transfer conditions.
Definition
sodium cyanide: A white orcolourless crystalline solid, NaCN,deliquescent, soluble in water and inliquid ammonia, and slightly solublein ethanol; cubic; m.p. 564°C; b.p.1496°C. Sodium cyanide is now madeby absorbing hydrogen cyanide insodium hydroxide or sodium carbonatesolution. The compound is extremelypoisonous because it reacts with the iron in haemoglobin in theblood, so preventing oxygen reachingthe tissues of the body. It is used inthe extraction of precious metals andin electroplating industries. Aqueoussolutions are alkaline due to salt hydrolysis.
Production Methods
Sodium cyanide was first prepared in 1834 by heating Prussian Blue, a mixture of cyanogen compounds of iron, and sodium carbonate and extracting sodium cyanide from the cooled mixture using alcohol. Sodium cyanide remained a laboratory curiosity until 1887, when a process was patented for the extraction of gold and silver ores by means of a dilute solution of cyanide.
Preparation
Sodium cyanide can be prepared by several methods (See Potassium Cyanide).
It is prepared by passing hydrogen cyanide through a 50% aqueous solution of sodium hydroxide followed by evaporation of the solution in vacuum: NaOH + HCN → NaCN + H2O
Another method is to reduce sodamide with carbon at red heat: NaNH2 + C → NaCN + H2↑
Also, sodium cyanide can be made by heating a mixture of sodium carbonate and carbon with ammonia at high temperatures: Na2CO3 + 4C + 2NH3 → 2NaCN + 3CO↑ + 3H2↑.
Reactions
Sodium cyanide, NaCN, white solid, soluble, very poisonous, formed (1) by reaction of sodamide and carbon at high temperature, (2) by reaction of calcium cyanamide and sodium chloride at high temperature, reacts in dilute solution in air with gold or silver to form soluble sodium gold or silver cyanide, and used for this purpose in the cyanide process for recovery of gold. The percentage of available cyanide is greater than in potassium cyanide previously used. Used as a source of cyanide, and for hydrocyanic acid.
General Description
A clear colorless aqueous solution.
Air & Water Reactions
Slowly evolves flammable and poisonous hydrogen cyanide gas.
Reactivity Profile
Sodium cyanide is weakly basic. Reacts with acids of all kinds to generate quantities of very poisonous hydrogen cyanide gas. Incompatible with strong oxidizing agents, especially if solution dries out. Gives insoluble products with silver(I), mercury(I) and lead(II) ions that may decompose violently under certain conditions.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Sodium cyanide is a white crystalline solid that is odorless when dry, but emits a slight odor of hydrogen cyanide in damp air. It is slightly soluble in ethanol and formamide. It is very poisonous. It explodes if melted with nitrite or chlorate at about 450°F. It produces a violent reaction with magnesium, nitrites, nitrates, and nitric acid. On contact with acid, acid fumes, water, or steam, it produces toxic and flammable vapors. Synonyms for sodium cyanide are hydrocyanic acid, sodium salt, and cyanide of sodium.
Health Hazard
Sodium cyanide is a highly poisonous compound by oral intake and by ocular and skin absorption. Accidental ingestion of a small quantity; as low as 100–150 mg could result in immediate collapse and instantaneous death in humans. At a lower dosage it can cause nausea, vomiting, hallucination, headache, and weakness. The toxicology of NaCN is the same as that of HCN. The metal cyanide forms HCN rapidly in the body, causing immediate death from a high dosage.
The lethal effect from cyanide poisoning varied with species. Investigating the acute oral toxicity of sodium cyanide in birds, Wiemeyer et al. (1986) observed that the LD50 values for the flesh-eating birds were lower than that for the birds that fed on plant material; vulture 4.8 mg/kg versus chicken 21 mg/kg. In a study on marine species, Pavicic and Pihlar (1983) found that at 10 ppm concentration of NaCN, invertebrates were more sensitive than fishes. In animals, the lethal dose of NaCN were in the same range by different toxic routes. A dose of 8 mg NaCN/kg resulted in ataxia, immobilization, and death in coyotes (Sterner 1979); however, the lethal time was longer, at 18 minutes.
Ballantyne (1983b) studied the acute lethal toxicity of sodium and other cyanides by ocular route. He found that cyanide instilled into the eye was absorbed across conjunctival blood vessels causing systemic toxicity and death within 3–12 minutes of the eye being contaminated. The toxicity of the cyanide did not decrease by mixing the solid with an inert powder such as kaolin.
LD50 value, intraperitoneal (mice): 4.3 mg/kg
LD50 value, oral (rats): 6.4 mg/kg
Sodium cyanide is a teratogen, causing fetus damage and developmental abnormalities in the cardiovascular system in hamsters (NIOSH 1986).
Sodium cyanide reacts with acids to form highly toxic hydrogen cyanide. There could be a slow liberation of HCN in contact with water.
Flammability and Explosibility
Sodium cyanide and potassium cyanide are noncombustible solids. Reaction with acids liberates flammable HCN.
Industrial uses
sodium cyanide and other water-soluble cyanides are used as modifying reagents for selective flotation of ores containing galena, sphalerite and gangue minerals.
Safety Profile
A deadly human poison by ingestion. A deadly experimental poison by ingestion, intraperitoneal, subcutaneous, intravenous, parenteral, intramuscular, and ocular routes. An experimental teratogen. Human systemic effects by ingestion: hallucinations, dstorted perceptions, muscle weakness, and gastritis. Experimental reproductive effects. hydrocyanic acid physiologically, inhibiting tissue oxidation and causing death through asphyxia. Cyanogen is probably as toxic as hydrocyanic acid; the nitriles are generally considered somewhat less toxic, probably because of their lower volathty. The nonvolaule cyanide salts appear to be relatively nonhazardous systemically, so long as they are not ingested and care is taken to prevent the formation of hydrocyanic acid. Workers, such as electroplaters and picklers, who are daily exposed to cyanide solutions may develop a “cyanide” rash, characterized by itching and by macular, papular, and vesicular eruptions. Frequently there is secondary infection. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes. moisture, acid. Many cyanides evolve hydrocyanic acid rather easily. This is a flammable gas and is highly toxic. Carbon dioxide from the air is sufficiently acidc to liberate hydrocyanic acid from cyanide solutions. Explodes if melted with nitrite or chlorate @ about 450”. Violent reaction with F2, Mg, nitrates, HNO3, nitrites. Upon contact with acid, acid fumes, water, or steam, it wdl produce toxic and flammable vapors of CNand NanO. Used in the extraction of gold and silver ores, in electroplating, and in insecticides. See also CYANIDE and HYDROCYANIC ACID, The volaule cyanides resemble Flammable by chemical reaction with heat,
Potential Exposure
Sodium cyanide is used as a solid or in solution to extract metal ores, in electroplating and metal cleaning baths; in metal hardening; in treatment of rabbit and rat burrows and holes and termite nests; in insecticides
storage
In particular, work with cyanides should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Cyanide salts should be stored in a cool, dry location, separated from acids.
Shipping
UN1689 Sodium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Sodium cyanide decomposes on contact with acids, acid salts, water, moisture, alcohols, and carbon dioxide, releasing highly toxic and flammable hydrogen cyanide gas. Aqueous solution is a strong base; it reacts violently with acid and is corrosive. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Absorbs moisture from the air forming a corrosive syrup. Corrosive to active metals, such as aluminum, copper, and zinc. Under acid conditions, sarin hydrolyzes to form hydrofluoric acid.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water.
Properties of Sodium cyanide
| Melting point: | 563.7 °C(lit.) |
| Boiling point: | 1497°C |
| Density | 1.6 |
| vapor density | 1.7 (vs air) |
| vapor pressure | 1 mm Hg ( 817 °C) |
| Flash point: | 1500°C |
| storage temp. | Poison room |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | White solid |
| pka | 9.36[at 20 ℃] |
| color | White |
| Odor | The dry salts are odorless, but reaction with atmospheric moisture produces HCN,
whose bitter almond odor is detectable at 1 to 5 ppm; however, 20 to 60% of the
population are reported to be unable to detect the odor of HCN. |
| PH | 11.7 (100g/l, H2O, 20°C) |
| PH Range | 11-12 |
| Water Solubility | 37 g/100mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8605 |
| BRN | 3587243 |
| Exposure limits | TLV-TWA (measured as CN) skin 5 mg CN/m3 (ACGIH and OSHA), 5 mg CN/m3/ 10-minute ceiling (NIOSH). |
| Dielectric constant | 7.6(Ambient) |
| Stability: | hygroscopic |
| CAS DataBase Reference | 143-33-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium cyanide(143-33-9) |
| EPA Substance Registry System | Sodium cyanide (143-33-9) |
Safety information for Sodium cyanide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Sodium cyanide
Sodium cyanide manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


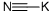
You may like
-
 Sodium cyanide, granules CAS 143-33-9View Details
Sodium cyanide, granules CAS 143-33-9View Details
143-33-9 -
 Sodium Cyanide CASView Details
Sodium Cyanide CASView Details -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
