SODIUM COBALTINITRITE
Synonym(s):Cobalt(III) sodium nitrite, Sodium cobaltinitrite;Sodium cobaltnitrite;Sodium hexanitritocobaltate(III);Sodium hexanitrocobaltate(III)
- CAS NO.:13600-98-1
- Empirical Formula: CoN6NaO12(-2)
- Molecular Weight: 357.95
- MDL number: MFCD00003512
- EINECS: 237-077-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
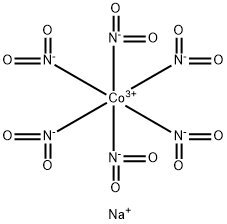
What is SODIUM COBALTINITRITE?
Chemical properties
SODIUM COBALTINITRITE is deep yellow to brown crystalline powder
The Uses of SODIUM COBALTINITRITE
SODIUM COBALTINITRITE is used in the detection Potassium.
The Uses of SODIUM COBALTINITRITE
Nitrosation reagent for the conversion of aromatic amines to 1,3-diaryltriazenes with excellent yield.1
The Uses of SODIUM COBALTINITRITE
For the detection of potassium with which it forms a slightly soluble compound.
What are the applications of Application
Sodium hexanitrocobaltate(III) is a nitrosation reagent
Preparation
Sodiumhexanitrocobaltate(III) ("sodiumcobaltinitrite") Na3Co(N02)6
is a well-known orange solid which precipitates potassium ions from aqueous solution as
K3Co(NO2)6. It is prepared by blowing air through a mixture of cobalt(II) nitrate and
sodium nitrite solutions in the presence of acetic acid:
2[Co(H20)6]2+ +12NO2- +2CH3COOH + 1/2O2 → 2[Co(NO)2)6]3- +2CH3COO-+ 13H2O
after filtration of any precipitated potassium or ammonium salts, the sodium salt is precipitated
by the addition of ethanol.
Purification Methods
Dissolve the salt (ca 60g) in H2O (300mL), filter to obtain a clear solution, add 96% EtOH (250mL) with vigorous stirring. Allow the precipitate to settle for 2hours, filter it off, wash it with EtOH (4 x 25mL), twice with Et2O and dry it in air [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1541 1965]. It forms yellow to brown crystals which are very soluble in H2O, are decomposed by acid and form an insoluble K salt. Used for estimating potassium.
Properties of SODIUM COBALTINITRITE
| Melting point: | 220 °C (dec.)(lit.) |
| storage temp. | Store below +30°C. |
| solubility | 720g/l |
| form | Solid |
| color | Orange to orange-brown |
| Odor | Odorless |
| PH | 5 (100g/l, H2O, 20℃) |
| Water Solubility | Soluble in water |
| Merck | 13,8677 |
| Stability: | Stable. Contact with conbustible material may cause fire. Incompatible with strong acids, organic materials, amines. |
| CAS DataBase Reference | 13600-98-1(CAS DataBase Reference) |
Safety information for SODIUM COBALTINITRITE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for SODIUM COBALTINITRITE
SODIUM COBALTINITRITE manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 SODIUM COBALTINITRITE 98%View Details
SODIUM COBALTINITRITE 98%View Details -
 Sodium hexanitrocobaltate 99%View Details
Sodium hexanitrocobaltate 99%View Details -
 Sodium Cobaltinitrite extrapure AR CAS 13600-98-1View Details
Sodium Cobaltinitrite extrapure AR CAS 13600-98-1View Details
13600-98-1 -
 Sodium cobaltinitrite AR,ACS CAS 13600-98-1View Details
Sodium cobaltinitrite AR,ACS CAS 13600-98-1View Details
13600-98-1 -
 Sodium CobaltinitriteView Details
Sodium CobaltinitriteView Details
13600-98-1 -
 Sodium Cobaltinitrite (CAS Number: 13600-98-1)View Details
Sodium Cobaltinitrite (CAS Number: 13600-98-1)View Details
13600-98-1 -
 Sodium Cobaltinitrite, PowderView Details
Sodium Cobaltinitrite, PowderView Details
13600-98-1 -
 SODIUM COBALT NITRITE, PowderView Details
SODIUM COBALT NITRITE, PowderView Details
13600-98-1
