Sodium aluminium hydride
Synonym(s):Sodium tetrahydroaluminate
- CAS NO.:13770-96-2
- Empirical Formula: Na.AlH4
- Molecular Weight: 54
- MDL number: MFCD00192443
- EINECS: 237-400-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
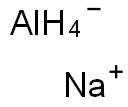
What is Sodium aluminium hydride?
Description
When sodium tetrahydroaluminate (NaAlH4) containing a small amount of titanium is heated, it takes in hydrogen gas, which can be released later by heating the solid again. Sodium tetrahydroaluminate has been studied as a prototype material for hydrogen storage, a key requirement for hydrogen-powered vehicles and other alternative energy applications.
Chemical properties
Sodium aluminum hydride is a white to light grey crystalline powder, which reacts with water, but is soluble in THF. Sodium tetrahydridoaluminate, sodium aluminum hydride, sodium alanate, NaAlH4, is a white, crystalline substance, mp 178℃; it decomposes at 210℃, liberating hydrogen and initially forming Na3AlH6. Sodium tetrahydridoaluminate is insoluble in hydrocarbons and diethyl ether. Its chemical properties resemble those of lithium aluminum hydride. However, the strong polar character of sodium aluminum hydride promotes side reactions during some reductions.
The Uses of Sodium aluminium hydride
Reducing agent similar to lithium aluminum hydride.
The Uses of Sodium aluminium hydride
Sodium Aluminum Hydride is used as a hydrogen (or heat) storage system. They have the advantage of having high storage capacity combined with low cost.
Preparation
Sodium aluminium hydride can be synthesized by reacting powdered sodium hydride and aluminium with hydrogen at 200 °C under high pressure (<100 atm). A catalyst, like triethylaluminium is used.
Definition
ChEBI: Sodium tetrahydroaluminate is an inorganic sodium salt.
General Description
A white crystalline solid. Density 1.24 g / cm3. If spread over a large flat combustible area, friction can ignite the material. Soluble in tetrahydrofuran.
Air & Water Reactions
Highly flammable. Does not react in dry air at room temperature. Very sensitive to moisture. Ignites or explodes on contact with water or moist air [NSC Nat. Safety News 77(2):37-40 1958]. Reacts with water to form sodium hydroxide, a corrosive material and hydrogen, a flammable gas. The heat of reaction may be sufficient to ignite the hydrogen.
Reactivity Profile
Sodium aluminium hydride is a strong reducing agent. Similar in reactivity to lithium aluminum hydride. May react violently with oxidizing agents. An explosion occurred during its preparation from sodium and aluminum in a medium of tetrahydrofuran [Chem. Eng. News 39(40):57 1961]. At elevated temperatures, the hydride reduces carbon dioxide or sodium hydrogen carbonate to methane and ethane. These gases are the explosive products formed when CO2 extinguishers have been used during hydride fires. 1:1 complexes with ether or dimethylamine and various tetrazoles are explosive. Tetrazoles include, 2-methyl, 2-ethyl, 5-ethyl, 2-methyl-5-vinyl, 5-amino-2-ethyl, etc. [US Pat. 3 396 170, 1968].
Hazard
Severe fire and explosion risk in contact with oxidizing agents and water, forms caustic and irritating compounds.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Safety Profile
Flammable when exposed to heat or flame. May ignite and explode on contact with water. Reacts violently with tetrahydrofuran when heated. When heated to decomposition it emits toxic fumes of NanO. See also ALUMINUM COMPOUNDS and HYDRIDES.
Potential Exposure
Used in chemical synthesis
Shipping
UN2835 Sodium aluminum hydride, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material.
Incompatibilities
A strong reducing agent. Very sensitive to moisture; reaction with water may cause fire or explosion. Also incompatible with iron, aluminum, and zinc in the presence of water. Hydrides are incompatible with acids, alcohols, amines, and aldehydes. Violent reaction, possibly explosive, on contact with water, air, oxidizers, acids, alcohols, and ethers. May ignite spontaneously in moist air. Does not react in dry air at room temperature. Reacts with water to form corrosive NaOH and flammable hydrogen gas. The heat of reaction may be sufficient to ignite.
Properties of Sodium aluminium hydride
| Melting point: | 178 °C |
| Density | 0.905 g/mL at 25 °C |
| Flash point: | -10 °C |
| storage temp. | water-free area |
| solubility | insoluble in ethyl ether; soluble in tetrahydrofuran |
| color | white hygroscopic solid |
| EPA Substance Registry System | Aluminate(1-), tetrahydro-, sodium, (T-4)- (13770-96-2) |
Safety information for Sodium aluminium hydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium aluminium hydride
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
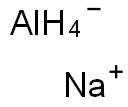
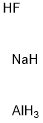

You may like
-
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Phenylephrine HCl 61-76-7 98%View Details
Phenylephrine HCl 61-76-7 98%View Details
61-76-7 -
 211915-06-9 98%View Details
211915-06-9 98%View Details
211915-06-9 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 201530-41-8 Deferasirox 98%View Details
201530-41-8 Deferasirox 98%View Details
201530-41-8 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
