Sodium aluminate
Synonym(s):Aluminum sodium oxide;Sodium aluminum oxide
- CAS NO.:11138-49-1
- Empirical Formula: AlNaO2
- Molecular Weight: 81.97
- MDL number: MFCD00085355
- EINECS: 234-391-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
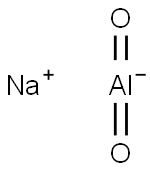
What is Sodium aluminate?
Chemical properties
Sodium aluminate is a white crystalline solid or solution.
The Uses of Sodium aluminate
Sodium aluminate is used in the treatment of industrial and municipal water supplies. It is also an effective precipitant for soluble phosphate in sewage and is especially useful in wastewater having low alkalinity.
Large quantities of sodium aluminate are used in papermaking where it improves sizing, filler retention,and pitch deposition.The addition of sodium aluminate to titanium dioxide paint pigment improves the nonchalking performance of outdoor paints.
Sodium aluminate is widely used in the preparation of alumina-based catalysts. Aluminosilicate can be prepared by impregnating silica gel with alumina obtained from sodium aluminate and aluminum sulfate. Reaction of sodium aluminate with silica or silicates has produced porous crystalline aluminosilicates which are useful as adsorbents and catalyst support materials, ie, molecular sieves.
The Uses of Sodium aluminate
Sodium Aluminate is used in method for producing Zeolite with enhanced Silicon/Aluminum.
What are the applications of Application
Sodium aluminate is an inorganic reagent useful as a catalyst for the isomerization of alkenes and amines.
Definition
sodium aluminate: white solid, NaAlO2 or Na2Al2O4, which is insolublein ethanol and soluble in water giving strongly alkaline solutions;m.p. 1800°C. It is manufactured by heating bauxite with sodium carbonate and extracting the residue with water, or it may be prepared in the laboratory by adding excess aluminium to hot concentrated sodium hydroxide. In solutionthe ion Al(OH)4- predominates.Sodium aluminate is used as amordant, in the production of zeolites,in effluent treatment, in glass manufacture, and in cleansing compounds.
Preparation
Small amounts of sodium aluminate are prepared in the lab by fusion of equimolar quantities of sodium carbonate and aluminum acetate, Al(C2H3O2)3, at 800°C. Other methods involve reaction of sodium hydroxide with amorphous alumina or aluminum metal.Commercial quantities of sodium aluminate are made from hydrated alumina, in the form of aluminum hydroxy oxide, AlO(OH), or aluminum hydroxide, Al(OH)3, a product of the Bayer process which is used to refine bauxite, the principal aluminum ore.
Commercial grades of sodium aluminate are obtained by digestion of aluminum trihydroxide in aqueous caustic at atmospheric pressure and near the boiling temperature.
Reactions
Sodium aluminate, NaAlO2, white solid, (1) by reaction of aluminum hydroxide and NaOH solution, (2) by fusion of aluminum oxide and sodium carbonate, the solution reacts with CO2 to form aluminum hydroxide.
General Description
Water solution of the white crystalline powder. Corrosive to metals and tissue.
Air & Water Reactions
Sodium aluminate will dissolve in water and produce a strong corrosive alkaline solution. May generate heat when water is added.
Reactivity Profile
SODIUM ALUMINATE generates a strong base in water; reacts violently with acids and corrosive to metals. Not compatible with copper, tin, zinc, aluminum, acids, phosphorus, or chlorocarbons.
Hazard
(Solution) Strong irritant to tissue.
Health Hazard
Material is caustic. Irritates skin, eyes, and gastrointestinal tract, causing redness of skin and eyes, burning sensation of mucous membranes.
Fire Hazard
Behavior in Fire: Containers may burst when exposed to heat.
Flammability and Explosibility
Non flammable
Safety Profile
Moderate irritant to skin, eyes, and mucous membranes. A corrosive substance. When heated to decomposition it emits toxic fumes of NazO.
Potential Exposure
Used in water and waste treatment; papermaking industry; in printing on fabrics; in the manufacture of pigments, milk glass, and soap; hardening building stone; sizing paper; as a water softener.
Shipping
UN2812 Sodium aluminate, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1819 Sodium aluminate, solution, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
The aqueous solution is a strong base. Reacts violently with acid. Incompatible with organic anhydrides; isocyanates, alkylene oxides; epichlorohydrin, aldehydes, alcohols, glycols, caprolactum, chlorocarbons. Corrosive to metals; attacks copper, tin, aluminum, and zinc.
Properties of Sodium aluminate
| Density | 3.240 |
| form | powder |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 11138-49-1(CAS DataBase Reference) |
| EPA Substance Registry System | Aluminum sodium oxide (11138-49-1) |
Safety information for Sodium aluminate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium aluminate
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

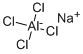
You may like
-
 Sodium Aluminate 99%View Details
Sodium Aluminate 99%View Details -
 Sodium aluminate CAS 11138-49-1View Details
Sodium aluminate CAS 11138-49-1View Details
11138-49-1 -
 Sodium aluminate CAS 11138-49-1View Details
Sodium aluminate CAS 11138-49-1View Details
11138-49-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
